Generative Medicine Series: Strategy and Layout of Biomedical Startups in the Clinical Application Field of Mesenchymal Stem Cells (Part 2)
An in-depth overview of the Mesenchymal Stem Cell (MSC) market
Hi guy, welcome to Part 2 of our article!
In Part 1, we introduced the concepts of mesenchymal stem cell (MSC) and mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) and explored the market potential in this field. I believe you now have a better understanding of the industry chain as we discussed in Part 1. In this part, we will continue the discussion by covering sections 5 to 9.

After reading this article, you will:
- Gain knowledge about regenerative medicine, specifically focusing on MSC (Mesenchymal Stem Cells) and MSC-EV (Extracellular Vesicles).
- Obtain a comprehensive overview of the stem cell industry chain, including identifying pain points and opportunities in the upstream, middle stream, and lower stream.
- Acquire marketing insights for developing future strategies to enter the MSC market.
So, let's dive in and get started!
Stem Cell Therapy Value Chain- Pain Points/ Potential Solution Overview
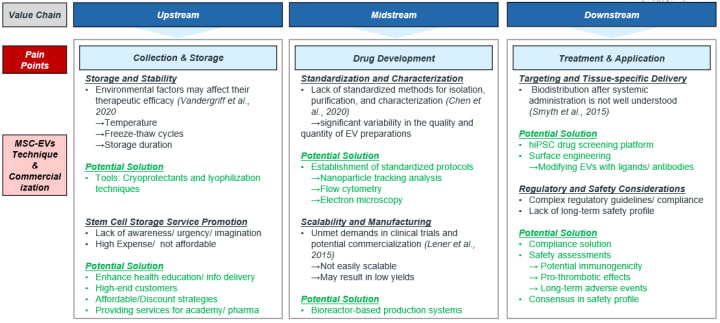
In the current industry chain, there are pain points that I would like to address using the example of mesenchymal stem cell-derived extracellular vesicles (MSC-EVs). The reason is that they have shown greater potential in certain aspects compared to mesenchymal stem cells themselves, and the regulatory aspects are relatively less stringent.
Pain Points and Solutions in Upstream
1. Storage and stability: Quality issue is a challenging obstacle that is influenced by environmental factors such as temperature, thawing techniques, and storage duration.
Strategy: Protecting cryoprotectants, freeze-drying techniques.
2. Effective promotion of storage services: Currently lacking of awareness among consumers, and its high costs/unaffordability is a big issue.
Strategy: Increase health education, target high-end customer groups, or adopt a low-cost strategy to address this issue.
Pain Points and Solutions in Midstream
1. Standardization and characterization: Lack of standardized methods for isolation, purification, and identification, leading to quantity and quality disparities.
Strategy: Establish SOP/protocols, require flow cytometers, electron microscopes, and other equipments.
2. Mass production: Lack of stability and need to improve commercialization should be focused.
Strategy: Batch systems with bioreactors are required to monitor or optimize the process.
Pain Points and Solutions in Downstream
1. Mechanisms of targeting and tissue delivery: Further clarification of mechanisms is required for the application of treatment.
Strategy: Tools of hiPSC drugs screening platform, and biological engineering techniques involving ligand/antibody modification of extracellular vesicles have the potential to understand the mechanism behind the sceen. (Note: More information about hiPSC drugs screening platform and other advanced applications will be discussed in another article)
2. Regulatory considerations: it involves addressing compliance requirements and streamlining the process of achieving regulatory approval.
Strategy: Establish compliance requirements, including to establish comprehensive compliance plans, seek expert advice, and develop optimization strategies by utilizing qualified materials.
3. Safety considerations: Currently lacking of long-term safety profiles is still an issue, which makes confusion in general assessment.
Strategy: Engaging healthcare KOLs and reach a consensus to define safety profiles, such as the duration and extent of adverse events could help the assessment of safety concerns.
Stem Cell Therapy Value Chain- a Closer Look in Upstream Opportunity
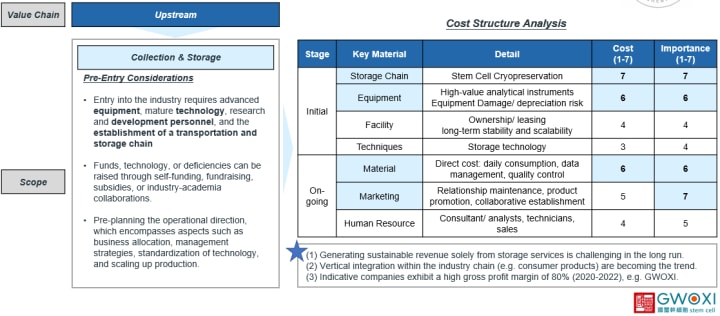
Now, let's take a deeper look and see what we should pre-considered before entering the market.
Pre-Entry Considerations
- Key factors: the establishment of equipment, technology, research and development personnel, and a robust cold chain for transportation is essential to the field.
- Fundings: the availability of funding sources.
- The construction of operational strategies: location, management approach, standardization of technology, and expansion of production. (commercialization)
Cost Structure Analysis
Here, I have conducted a simple cost structure analysis for various aspects, including technology, equipment, personnel, etc. The scoring ranges from 1 to 7, 1 point represents the least important/least expensive, while 7 points presents the most important/most expensive. The scoring is based on desk research results, considering factors such as funding, entry barriers, and our industry insights, it's a reference for your decision.
Stage & Key Materials
First, I divided the costs into initial expenses and ongoing expenses. The key materials including storage chain, equipment, facility, techniques, material, marketing, and human resources, each definition and their scoring reasons are as following.
Details in Initial Stage
- Storage Chain: It includes the crucial aspects of freezing, transportation, and storage. Due to the process from 0 to 1, the establishment cost and importance of this category receive the highest score.
- Equipment: The equipment category includes high-priced freezing equipment, considering the factors like depreciation and damages, ranking second.
- The facility: It encompasses rental or self-owned premises and specifications for mass production.
- Techniques: The aspect focuses on its storage techniques.
Details in Ongoing Expenses
- Material: Including the consumables/fixed resources, daily maintenance, and quality control, etc.
- Marketing: Which contains relationship maintenance, product promotion, partnership establishment, etc. Its importance lies in its ability to enhance efficiency and provide opportunities for collaborations.
- Human resources: Consultants, technicians, sales personnel, etc. Considering the factors of funds, entry obstacles, it is important to note that a lower score does not necessarily indicate a lack of significance.
Key Insight
- It is challenging to achieve long-term benefits through storage services at the current stage, as consumer awareness has yet to reach its peak. The practical application of stem cells, although promising, still requires further development.
- Integration of the upstream, midstream, and downstream sectors is a growing trend.
- High-profit opportunities are indicators of success. Taking GWOXI as an example, their gross profit margin has exceeded 80% for three consecutive years, showcasing commendable performance.
Stem Cell Therapy Value Chain- a Closer Look in Midstream Opportunity
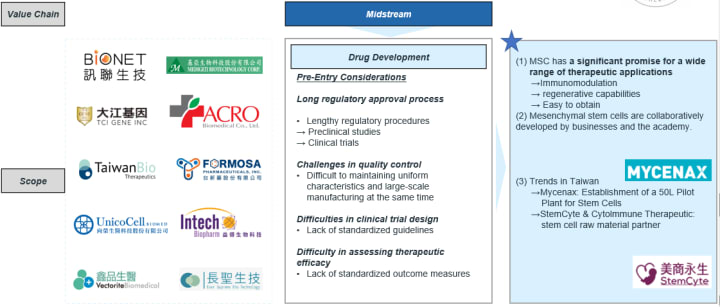
Pre-Entry Considerations
- The time cost is high, but the success rate is not necessarily high.
- Quality management needs to be maintained.
- Clinical trial design models need to be optimized
- It is challenging to evaluate efficacy.
Key observations
- Mesenchymal stem cells (MSCs) are currently the most widely used due to their immunomodulatory and regenerative properties, as well as their easy accessibility.
- Industry-academia collaboration is a growing trend.
- Trends in Asia (takes Taiwan as an example)
- Mycenax established a 50L stem cell pilot plant
- Stemcyte and CytoImmune have become cell raw material supply partners
- Collaboration between BioNet & Shan Medical University Hospital
- Collaboration between Medigen Biotech Corp. & Mackay Memorial Hospital
Stem Cell Therapy Value Chain- a Closer Look in Downstream Opportunity

Pre-Entry Considerations
- High entry barriers
- High profitability
- Government support
- Regulatory relaxation
- Asian trends (takes Taiwan as an example)
- Cllaboration between TCI Gene & Qisda
- UnicoCell: Phase III adipose stem cell drug
Current Opportunities
- There are limitations in the development of pharmaceutical treatments, and stem cell therapy offers a promising future.
- Compared to pharmaceuticals, stem cell therapy has the opportunity to enter the application stage earlier in Phase II (for some countries).
- Precision treatment: It has the advantage of better targeting, potentially providing improved therapies.
- Expanding market size: Regenerative medicine is already a global trend, and the market will only continue to grow.
Conclusion Summary
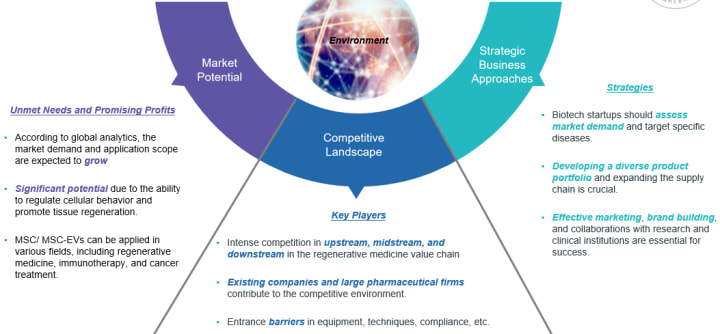
- Market potential: Unsatisfied demand, tremendous opportunities, and the significant potential in diverse applications are seen.
- Competitive landscape: Intense competition among upstream, midstream, and downstream players, dominance of large international companies, and entry barriers related to technology, equipment, and compliance are the entry obstacles.
- Business strategy: Demand assessment, developing a promising product portfolio, conduct efficient marketing strategies, and reputation building are key paths to achieve success.
If you enjoy our article, we kindly ask you to consider subscribing or showing your support. For a comprehensive report download, please visit our Patreon page. For more information, please refer to the following link: P.Ambrose.article. Thank you.
About the Creator
P. Ambrose
A 25-year-old analyst. I share articles of sports, technology, pharmaceuticals, and personal experiences.
Personal Link: https://linktr.ee/P.Ambrose.article






Comments
There are no comments for this story
Be the first to respond and start the conversation.