Connecting the Dots: Oral Bacteria and Colorectal Cancer Risk
From Mouth to Gut
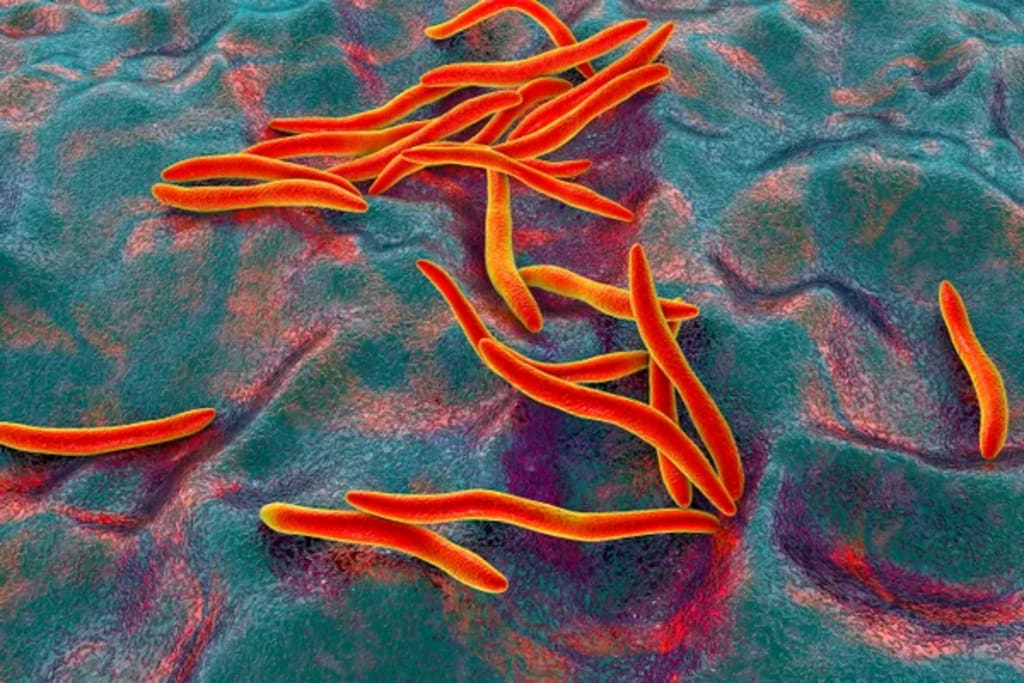
The bacteria known as Fusobacterium nucleatum live in the human mouth. But in the case of colon cancer or the rectum, the bacteria are found in tumors in the gut, where they assist cancer cells with getting away from the immune system and spread to different parts of the body.
In another study, a group of scientists from the Fred Hutchinson Cancer Center in the U.S. found a particular subtype of the bacterium is generally more prominent in colorectal cancer (CRC) tumors.
CRC is the seventh most common kind of cancer, where the number of cases rose by 20% from 2004 to 2014. Around the world, the overall CRC frequency has declined however, experts wrote in the journal Science last year, that the occurrence of age-adjusted early-onset CRC "has increased at a rate of 2-4% in many nations, with an increase in people younger than 30 years."
As per the group's experiments, described in a paper in Nature in March, a hereditary factor could be boosting Fusobacterium's ability to connect with cancer of the gut. The group also showed that when mice were infected with this kind of Fusobacterium, their intestines developed precancerous formations called adenomas.
Specialists said the study's findings could be used in the future to foster tests to identify CRC early and foster designated treatment choices.
It's very own clade
The scientists started by culturing Fusobacterium bacteria collected from 130 human CRC tumors in the laboratory. Then they planned the whole hereditary composition of the isolated bacteria and found that out of the four known Fusobacterium nucleatum subspecies, just Fusobacterium nucleatum animalis (Fna) was connected with CRC tumours.
Individual members from similar species have slightly different DNA. Pangenomic analysis assists researchers map all the genes as well as those parts of the genome that some but not all individuals from the species have. This part is known as the accessory genome. The individuals from a species can be further subclassified relying upon the accessory genomes they have.
In their analysis, the scientists found Fna has the smallest core genome (the part that all individuals from the species have), indicating there could be different subtypes of Fna. Accordingly, they followed the evolutionary history of the bacteria by following the changes in its genes. This analysis revealed that Fna, rather than being one homogenous group, is composed of microbes from two different evolutionary lineages.
Researchers call a group of life forms belonging to one evolutionary lineage a clade. Hence, the scientists recognized two unique clades of Fna: they called these Fna C1 and Fna C2. They further found Fna C2 bacteria are connected with CRC tumors and that they have extra genetic factors to help them in this regard.
Colonizing the gut
Both physical and hereditary contrasts between the two clades appeared to add to Fna C2 bacteria's ability to connect with CRC tumors. The Fna C2 bacteria looked longer and thinner than the Fna C1 bacteria. Such contrasts can influence how bacteria can live in host tissue as well as evade the body's immune system, the author wrote in their paper.
Hereditarily, Fna C2 bacteria had genes expected to munch two compounds for energy in the human gut: ethanolamine and 1,2-propanediol. These genes were missing in Fna C1. So the analysts concluded Fna C2 bacteria's ability to connect with CRC cancers was at least partly dependent upon them "having increased nutrient scavenging mechanism and improved metabolic potential".
The analysts approved their discoveries by analyzing genomes present in more than 1,200 human feces tests, generally 50% of which were from individuals with CRC while others were from healthy people. They found that the Fna genes expected to metabolize ethanolamine and 1,2-propanediol were more enhanced in feces tests from CRC patients than in examples from individuals without CRC.
Mouth to gut
Researchers recently accepted Fusobacterium bacteria could go from the mouth to the gut by infecting the bloodstream when, say, somebody brushed their gums excessively hard or during routine dental procedures. The authors of the new Nature paper pitched a new route: the bacteria might have descended through the gastrointestinal tract to arrive at the colon.
Bacteria don't follow this as a rule since they can't endure the exceptionally acidic environment of the stomach.
But, the scientists found Fna C2 could. These bacteria could grow in more acidic circumstances than Fna C1 bacteria, and they likewise had specific genes that could oppose the impacts of acids. These genes came online when the acidity was tantamount to that of stomach acid.
In mice as in humans
Then, the analysts examined whether Fna C2 could actuate the advancement of cancers in the gut. For this, they presented Fna C1 bacteria in the aggravated guts of certain mice and Fna C2 bacteria in the inflamed guts of others. (These mice are a typical creature model used to explore conditions that affect humans.) They found an essentially higher rate of adenomas in the intestines of the mice treated with Fna C2 bacteria.
They additionally noticed that the intestines of Fna C2 treated mice had different metabolic profile changes predictably with recently reported relationship between differential metabolite levels and cancer progression
"Generally, our outcomes show the capacity of Fna C2, however not Fna C1, to metabolically influence the intestinal milieu towards" conditions helpful for CRC, the authors wrote.
At last, the scientists tried their hypothesis in a cohor of human patients. Working with CRC tissue and non-carcinogenic tissues from similar individuals, the authors affirmed that Fna C2 was the main Fusobacterium subtype improved in CRC tissues. They found comparative outcomes in feces tests from those with CRC however not in those from healthy people.
The difficult road to clinical trials
According to Neetu Kalra, a cancer therapeutics specialist at Azim Premji University, Bhopal, "The review presents promising possibilities for the progressions of microbial cell therapy, which include the use of changed bacterial strains to control medicines into tumors straightforwardly."
Varun Aggarwala is an associate professor at Jio Foundation, Mumbai, who works with fecal transplants for infectious and inflammatory bowel diseases. He referred to the study as "comprehensive" and said, "Studies like this give a solid foundation to the broader community to configuration designated microbial intercessions and diagnostics for CRC."
He added that research should track the gut and oral microbiome of high-risk people and their growth microbiome after a CRC finding to comprehend how certain types of bacteria can cause cancer.
Likewise, Dr. Kalra said studies to come could look at the "colonization timetable" of Fna C2 bacteria: the CRC stage at which the bacteria become related to the tumors. "Assuming that colonization happens early," she explained, "it could work with early CRC diagnosis".
On the other side, she said developing a medication that could specifically target Fna C2 bacteria without affecting Fna C1 or other gut bacteria "presents a huge challenge".
About the Creator
shanmuga priya
I am passionate about writing.






Comments (1)
This is good information! Well done!