Content warning
This story may contain sensitive material or discuss topics that some readers may find distressing. Reader discretion is advised. The views and opinions expressed in this story are those of the author and do not necessarily reflect the official policy or position of Vocal.
WHO approves second, affordable vaccine in fight against deadly malaria
Health
WHO approves second, affordable vaccine in fight against deadly malaria
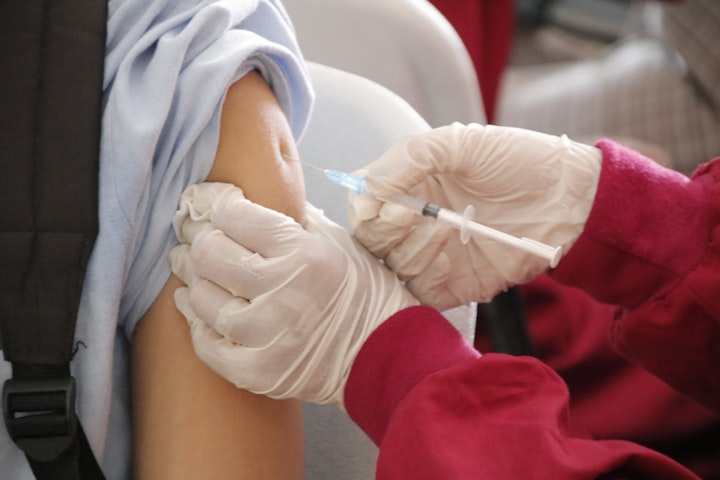
The World Health Organization has granted approval to a newly developed anti-malaria vaccine, providing countries with a cost-effective and easily accessible solution in combating one of the primary causes of child mortality in Africa.
Issued by Oxford University, the R21/Matrix-M vaccine is the second of its kind recommended by WHO, as stated in their official statement on Monday.
Data derived from preclinical and clinical trials led to the recommendation, revealing that the vaccine reduced symptomatic cases by 75 percent after a year-long three-dose series, particularly in regions with high seasonal malaria transmission. Oxford University also stated that the Phase III clinical trial results are currently undergoing peer review.
Costing between $2 and $4 per dosage, R21 represents a financially feasible intervention, as attested by WHO. They further mentioned that the vaccine is expected to be accessible by mid-2024.
Following the detection of rare malaria cases in Florida and Texas, it is crucial to understand more about the disease.
"The day we acquire a safe and effective malaria vaccine has always been my aspiration as a malaria researcher. And now, we have two," WHO Director-General Tedros Adhanom Ghebreyesus declared in the release. Malaria is a life-threatening yet treatable illness primarily transmitted through the bites of specific mosquitoes. Those afflicted with malaria often experience symptoms such as fever, chills, and flu-like signs.
Millions of cases are reported annually, with WHO reporting an estimated 619,000 malaria-related fatalities in 2021. The vast majority of malaria cases occur in Africa, where children under the age of 5 account for 80 percent of the reported deaths in the region. In the United States, the Centers for Disease Control and Prevention states that approximately 2,000 malaria cases are recorded each year, with the majority originating from overseas travel. Recent cases detected in Florida and Texas, involving individuals who had not traveled abroad, led health authorities to release warnings in July.
In 2021, WHO sanctioned the first malaria vaccine, known as RTS,S. However, while the demand for malaria vaccines is unprecedented, the supply of RTS,S remains limited, impeding prevention efforts according to WHO's statement on Monday. WHO, alongside UNICEF and Gavi, a global vaccine alliance, allocated 18 million doses of RTS,S to 12 African countries for the years 2023 to 2025.
The Serum Institute of India, responsible for manufacturing the latest R21 vaccine, claims to have the capacity to produce 100 million doses annually. The institute has long been a partner in supporting Oxford and served as the primary funder for the Phase III clinical trial, as confirmed by the university.
There is currently no evidence suggesting one vaccine outperforms the other, states the WHO, emphasizing the lack of a head-to-head trial involving the two vaccines. The Bill and Melinda Gates Foundation ceased direct financial support to RTS,S last year, as reported by the Associated Press, citing lower efficacy than desired. While public health experts applauded this breakthrough, they cautioned that more needs to be done in the ongoing fight against malaria.
NoThe R21/Matrix-M vaccine, produced by the Serum Institute of India, is set to manufacture over 100 million doses annually, with plans to increase production to 200 million doses per year, as reported by the BBC. Clinical trials have revealed that the R21 vaccine effectively reduces symptomatic malaria cases by 75% within a 12-month period following a three-dose regimen. Furthermore, the efficacy of the vaccine remains consistent when a fourth dose is administered one year after the third dose, paralleling the seasonal administration of the RTS,S vaccine.
nprofit organization Malaria No More UK's Executive Director of Advocacy and Strategy, Gareth Jenkins, stressed the significance of both vaccines receiving financial backing and support to promptly reach children in need. "Malaria financing globally is far from adequate, and deaths from malaria rose during the pandemic, surpassing pre-pandemic levels. Consequently, as new tools are developed, complacency is a luxury we cannot afford," he emphasized.
thanks for all
About the Creator
AMENA ISLAM
I derive great satisfaction from showcasing my artistic pursuits and written works,regardless of their emotional demeanor, whether they be whimsical or deeply meaningful. Additionally, I shall proudly present a comic strip currently. thanks
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments (1)
An interesting link between malaria and sickle cell: https://www.verywellhealth.com/sickle-cell-and-malaria-5323165 I learned about this in nursing school years ago and thought you might be interested, too....but you might already know, too.