The Worst Quality Control Fail of All Time
A pharmaceutical company is having to recall lots of lots of its product—birth-control pills—for a big-time packaging error! The big news is that this is not the first time this has happened!

When students ask me in my management classes if I have any career regrets, I don't go into a long rant over the things that coulda/woulda/shoulda happened over the years. No, as a strategic management professor and consultant who has reached AARP-eligibility, I don't have really any regrets about how my career ended up—save one. But yes, it's a big one! Yes, it would have meant that I would not have been standing in front of classrooms full of business students for over two decades and cranking out articles to survive in the "publish or perish" world in which we academics live.
So, what's the one job that I think of that I say: "Man, I wish I had taken that offer!" Well, there's one and only one for me. When I was a young pup graduating college in the mid-1980s, I had a series of interviews with a particular company that led to a job offer. Looking back, I can safely say that even I could have sold that product! Not only was the job being a representative selling a single product for a pharmaceutical company with one of the best, all-time brand name reputations and recognitions among all American companies, I would have been peddling a prescription drug that over the years has seen steady, almost unwavering demand. Yes, it's a product that basically does sell itself—and retains loyal customers for years of continuous use. Ah, the sales commissions! The bonuses! The trips to the Caribbean!
So what job opportunity do I regret passing up on that would likely have led me on a path where right now, I would be able to be retired, playing golf, and thinking about the exotic locale with fancy frozen drinks where my wife and I would be traveling next? Well, had I not passed on the opportunity in favoring of staying in school to get my MBA, I would have been the sales rep for a big part of Texas for a little company called Johnson & Johnson. And what "magic product" that basically sells itself to millions and millions of women annually would I have been representing? Well, I would have been working for J&J's subsidiary, Ortho Pharmaceutical, talking with doctors and pharmacists about the benefits of "birth control pills!" And no kids, at that time, there was no generic version of the product available and Ortho was the sole source of "the pill" thanks to its then-patent protection.
So yes, that "road not taken" would have likely led me to riches and success—and that's why that job is "the one that got away" that I still think about to this day. Oh well... Still, I must say that when I see a business news story that has to do with the birth control market, I do take note. However, this story is one that should capture the attention of all, as we have just seen perhaps what will go down in history as the worst quality control fail of all time. And yes, it involves "the pill!"
The Most Critical Quality Control Error Perhaps of All-time!

When we talk about and teach about quality control, all too often eyes glaze over in classes and students start to look really relaxed in their chairs. The basics of statistical quality control lie in math—and so that makes for dangerous territory for many students! In truth, "Six Sigma" might as well be nuclear physics to the majority of business students today! And when we talk about "defect rates" for widgets or even for "real" products like everything from cars to laptops and even hamburgers, the true impact of quality just seems like a "cost of doing business." Yes, management wants to have better quality in any product, but a bottle of shampoo that only contains 15.8 ounces of product instead of the labeled 16 or even a hamburger that is made with only two pickles instead of the prescribed four really doesn't have a serious impact on our lives.
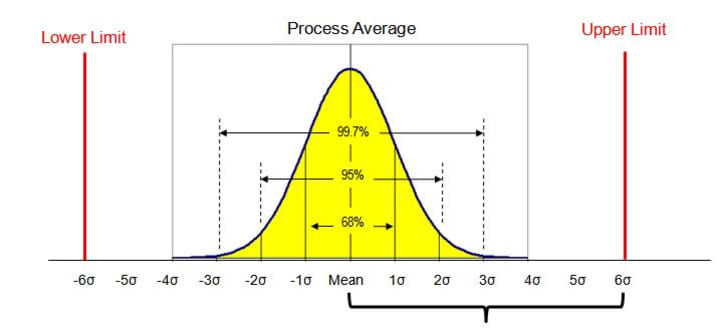
But if you think about quality control in really critical situations, life or death ones, the topic suddenly becomes far more important. And yes, one would think that the manufacturing and packaging of birth control pills is one where there would seemingly be absolutely no room for error. The consequences of a bad or adulterated (no pun intended...) product would just be too severe, right?
However, in recent days, the U.S. Food and Drug Administration (FDA) has issued an urgent recall for birth control pills that were packaged incorrectly, and if used in the order that they were packaged (which, let's face it, everyone would expect to do), a minor unintended health consequence—i.e. pregnancy—could occur! This has the makings of yes, a very serious situation, and yes, also a great case study in the ultimate importance of having proper quality control measures in place in any manufacturing operation. And sadly, believe it or not, this is not the first time such an "incident" has occurred! Talk about a case study that will pique your students' attention!
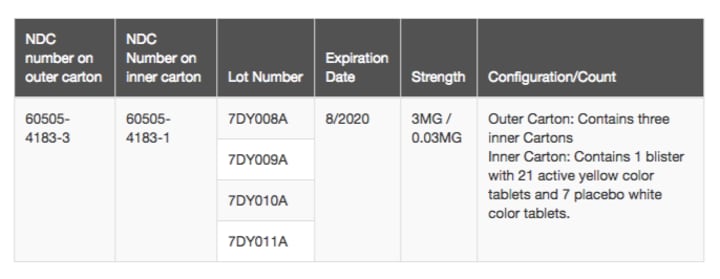
So, let's go over the basic facts here. In early March 2019, the FDA announced a recall of birth control pills produced by Florida-based Apotex. Never heard of them? Well, there's good reason, and that's because they don't make any of the branded pharmaceuticals that you see countless ads for today (I'm looking at you, advertised-for- everything Humira!). According to the company's website, "Apotex currently produces more than 300 generic pharmaceuticals in approximately 4000 dosages." In the United States alone, their catalog includes 483 separate generic drug offerings (See: Apotex: All Products). So, while you may never have heard of Apotex before today, the odds are pretty good that you have taken—or are currently taking—one of their prescription drugs.
Apotex's birth control pill that is the subject of the recall is the generic version of birth control pills that contain two active ingredients: Drospirenone and ethinyl estradiol. According to the FDA, these 28-day birth control pills are currently marketed under various brand names in the U.S., including:
- Ocella
- Safyral
- Syeda
- Yasmin
- Zarah
The description of the product from the company's catalog is shown below:

Source: Apotex (http://www1.apotex.com/products/us/default.asp?qt=All)
Sadly, the recall was necessitated by a packaging error—one of monumental proportions! Below, Katie Korte, who serves as the lead clinical pharmacist at Truman Medical Center in Kansas City, offers a very good overview of the problem with Apotex's packaging and what consumers need to know about the recall.
Of course, it must be stressed that this recall is extremely serious to those who are taking any generic birth control pills! And so if you are reading this article anytime in 2019, first and foremost it must be said that the FDA recommends that if you have any questions or concerns that your particular birth control prescription might be included in the recall effort, you should contact Apotex immediately. This can be done via phone at 1-800-706-5573 or by email at [email protected]. In the meantime, those who believe that they might have the "bad lots" of pills are advised by the FDA to talk with their pharmacist and to—most importantly—not stop taking their birth control pills, but also, to "use a non-hormonal method of birth control."
Recall Information on Apotex Birth Control Pills - March 2019
Source: U.S. Food and Drug Administration (https://www.fda.gov/Safety/Recalls/ucm632629.htm)
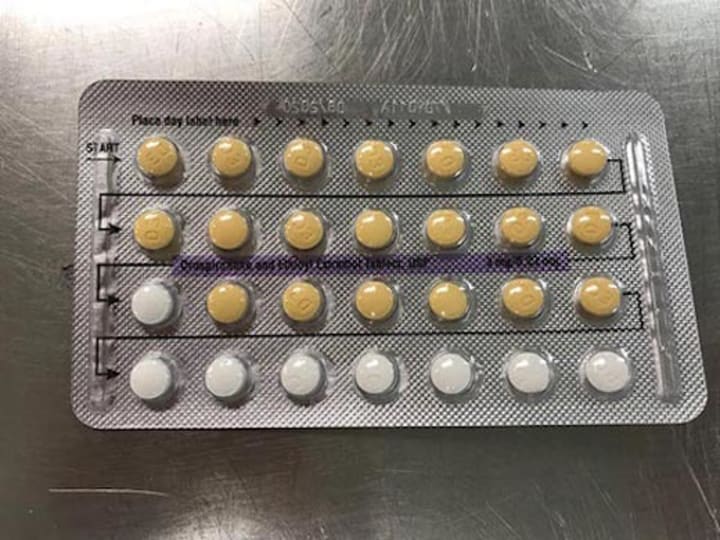
Many are familiar with the standard packaging of the 28-day birth control pill prescription. Clearly recognizable is the pattern of 21 pills of one type and 7 pills of another. The packaging makes it quite clear as to what pills for a woman to take, based on the day of the week they are starting their prescription. And it goes without saying, of course, that "order" is very important to the efficacy of this drug in preventing pregnancy.
Correct Packaging for a 28-day Birth Control Pill Cycle

Now, it is clear that the "quality control" at Apotex was—shall we say—lacking in the case of this particular drug. And of course, in the global economy in which we exist today, Apotex is based in Toronto, Canada and according to information the company supplied to the FDA, the birth control pills in question are actually produced by Oman Pharmaceutical Products Co. LLC. as a subcontractor to a German firm, Helm AG, based in Hamburg (Source: FDA: https://www.fda.gov/Safety/Recalls/ucm632629.htm). So yes, fingers will be pointed and the litigation will fly with the complex sourcing involved in this incident. However, in the context of the global pharmaceutical supply chain, there is nothing unusual about the production and distribution method that Apotex has employed with its birth control product.
Now, just how badly was the packaging done in the Omani factory? Well, you can see for yourself in the three samples provided by the FDA for consumers in their recall alert. There were packages found in which the 21-7 pattern was off by at least one pill...
Sample Mispackaged Apotex Birth Control Pills - March 2019
Source: U.S. Food and Drug Administration (https://www.fda.gov/Safety/Recalls/ucm632629.htm)
... And packages where there were simply 28 of the same kind of pill...
Sample Mispackaged Apotex Birth Control Pills - March 2019

Source: U.S. Food and Drug Administration (https://www.fda.gov/Safety/Recalls/ucm632629.htm)
... and then there were instances of pills missing from the blister packs.
Sample Mispackaged Apotex Birth Control Pills - March 2019

Source: U.S. Food and Drug Administration (https://www.fda.gov/Safety/Recalls/ucm632629.htm)
All in all, one can safely say that Apotex and its contractors were not even relatively close to Six Sigma quality on the manufacturing line for those four particular lots being recalled.
Now what is amazing to me, not as a "birth control expert" or a regular observer/analyst of the pharmaceutical marketplace, but as a management consultant and educator, is the fact that in this corner of the prescription drug market, quality control seems to be a continuous problem. The truly alarming thing is that this is not the first time that this particular company has had quality control problems that have led to recalls. And across just the family planning segment of the pharmaceutical industry, there have been other birth control pill recalls for similar issues. Talk about a major "D-oh!"
How bad are the quality control issues here for a product that yes, one just de facto would believe would have the highest quality standards? Just last year, in fact, Apotex had to issue a voluntary recall on its Alysena 21 and Alysena 28 birth control pills in Canada due to the fact that lots were found that contained chipped pills. This was undertaken as a response to the fact that both Health Canada (that country's equivalent of our FDA) and the Society of Obstetricians and Gynecologists of Canada warned that the chipped pills might not deliver the complete dose of the active drug ingredients that work to prevent pregnancies, greatly reducing the efficacy of the birth control pills.
And in a case eerily similar to the present recall, Apotex had to issue a recall in 2013 in Canada for the same kind of packaging error that prompted this year's recall! Instead of the 3 rows of 7 active pills (21 in all) and the single row of 7 inactive (placebo) pills, in the 2013 incident, the mix was 14 and 14—2 rows of 7 each! That could definitely lead to problems!
Now, perhaps even more shocking is the fact that packaging errors have been the cause of several pharmaceutical companies having to issue recalls for their birth control products just within the past decade! Just a cursory investigation of the subject (that means good "Googling" honestly!) found that Apotex was by no means alone in having some really severe quality issues in the packaging of their birth control pill products for their users. Allergen has been sued over its mispackaging of birth control products....
For instance, in 2011, Qualitest Pharmaceuticals, a division of Endo, had to issue a voluntary recall for multiple lots of their birth control pills when it was discovered that the order of active versus placebo pills had been reversed, and again, if women took them in the order in which they were wrongly packaged, an unintended pregnancy could occur. This, in turn, led to Qualitest being sued by over 100 women across 28 states claiming that their packaging error had led to unintended pregnancies, seeking support for children born due to the company's faulty packaging.j
As has industry giant Pfizer (for misplacing placebo pills with the active pills in its packaging).
And even branded birth control pills have not been exempt from having serious packaging mistakes. Late last year, Janssen Pharmaceuticals had to recall 3 lots of its popular Ortho-Novum pills due to mistaken instructions that were included with its product—instructions that, if followed, would have potentially led to unintended pregnancies!
Analysis
Okay, so after reading this, your reaction might be something like "Holy Mother of God!"—or worse! The point of all of this is not to scare the Bejesus out of many students or just casual readers perusing this article. But while the facts are indeed scary, they drive home a very important point. All too often, we throw around the well-worn—often too very well-worn—axiom that "quality matters." In this case—the quality of correctly packaging birth control pills does matter—greatly! Lives are changed, and yes, lives are created based on not having the proper quality controls in place to make certain—make that damn certain—that the product is packaged correct 100% of the time.
And so if you want a case study to illustrate to your students why quality is important, this—this is it! It is certainly surprising to me—both as a management consultant and professor and as just an average male consumer—that there has been such a stupefying packaging problem in such a "mission critical" product as birth control pills. For women taking birth control pills, however, I'm sure this article might just spur some checking in their purse or their medicine cabinet at home.
When I started researching this piece, I thought that the current news over the Apotex recall was an isolated incident. After all, we routinely hear in media about company after company in the pharmaceutical area having a product recalled. But when it is for something on the level of birth control pills, well, that gets one's attention—for many reasons! The point is not to urge folks to reconsider their birth control measures. However, given the quality issues that this sector of the pharmaceutical industry has seen in recent times over what would appear to be the easiest part of the efficacy equation—putting the right pills in the right order—one has to think a bit—perhaps even a big bit—differently after learning of these repeated quality problems.
So, in the end, while I may think about the road not taken in my own career for not taking the "birth control job" back in the day, I would not want to be a senior executive with any of the pharmaceutical companies involved in these instances of what is nothing less than sloppy, inexcusable mistakes not just in their packaging, but in having proper and sufficient quality control measures in place. Whether it was their own company or a contract manufacturer that actually made the error, as was likely the case with the most recent Apotex case, the truth of the matter is that in 2019, you must have the proper measures and controls in place to ensure that mistakes like these simply can't happen. In our age of 24/7 news and the power of social media, a company and its image can be taken down in an instant. And so now we live in an era where companies need to think about having Six Sigma quality in their operations yes for the sake of their customers, their employees, and their stakeholders, but also for their very survival in the marketplace. You just can't have serious product mistakes in an area such as birth control and expect to be successful over the long term. How hard can it really be folks—3 rows of 7 active pills followed by 1 row of 7 inactive pills?
About David Wyld
David Wyld ([email protected]) is a Professor of Strategic Management at Southeastern Louisiana University in Hammond, Louisiana. He is a management consultant, researcher/writer, publisher, executive educator, and experienced expert witness. He is the founder and publisher of both The IDEA Publishing (http://theideapublishing.com) [The Best in News, Information and Content Marketing] and Modern Business Press (http://modernbusinesspress.com) [The Best in Academic Journals].
David Wyld’s Online CV:https://clearvoice.com/cv/DavidWyld
Social Media Links to David Wyld:
Facebook:https://www.facebook.com/david.wyld
Twitter:https://twitter.com/PublisherMBP (@PublisherMBP)
LinkedIn:https://www.linkedin.com/in/david-wyld-4923707/

Professor David C. Wyld
Show your support for David and for the Vocal platform.
Like what you just read? Did it make a difference to you? If so, please see and share this article through social media, email, and even the old-fashioned way of printing it off for a colleague or friend!
And while you’re at it, ask yourself a simple question: Was the info worth a buck or two—or a whole lot more to you, your career, your company? If so, please consider “tipping” (after all, it’s the polite thing to do!) using the easy link below. In providing a small tip, you not only help support the author's work, but you help keep the unique platform that Vocal Media is building be an advertising-free environment—and don't we all need more of that to make our online experiences better today? Please consider showing your support below and voting for good, ad-free ideas on the web!

About the Creator
David Wyld
Professor, Consultant, Doer. Founder/Publisher of The IDEA Publishing (http://www.theideapublishing.com/) & Modern Business Press (http://www.modernbusinesspress.com)
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments
There are no comments for this story
Be the first to respond and start the conversation.