Carbon Monoxide Poisoning
How Carbon Monoxide Slowly Kills
Carbon monoxide poisoning is not typically on the forefront of people's minds on a daily basis. When considering the most commonly discussed dangerous topics in society, subjects like murder, terrorism, drugs, poverty, and disease often take precedence. These topics often dominate the discourse, reflecting their apparent significance to human mortality. In comparison, the threat of carbon monoxide poisoning is relatively low, with approximately 430 fatalities reported in the United States each year. However, despite its relatively low occurrence, carbon monoxide poisoning has found its place in popular culture, particularly in Hollywood. Despite its occasional portrayal in films and media, carbon monoxide remains poorly understood by the general public.
Carbon monoxide (CO) is a molecule consisting of a carbon atom triple-bonded to an oxygen atom. It is important to note that carbon monoxide should not be confused with carbon dioxide (CO2), a gaseous molecule produced as a result of metabolic processes, including respiration. Carbon dioxide is composed of a carbon atom double-bonded to two oxygen atoms. While these molecules share some similarities – both being gaseous at room temperature and having no color, taste, or smell – they exhibit crucial differences. The most significant distinction is that carbon dioxide is not toxic, whereas carbon monoxide is highly toxic when inhaled due to its profound impact on the function of hemoglobin.
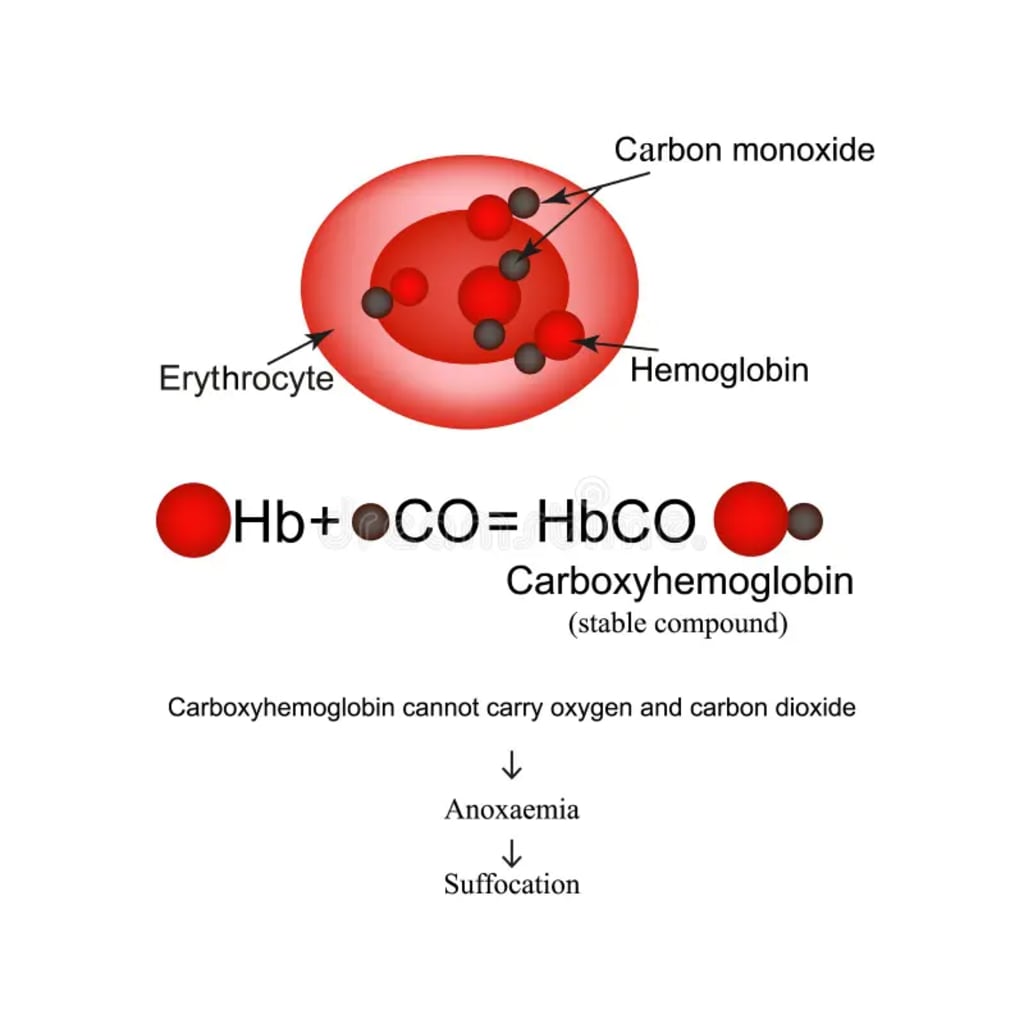
Hemoglobin is a vital protein found within red blood cells, and its primary role is to transport and release oxygen in the bloodstream. Hemoglobin contains four protein segments called heme groups, each featuring an iron atom at its core. When red blood cells circulate through the lungs, inhaled oxygen diffuses into the bloodstream and interacts with hemoglobin. Oxygen molecules bind to each heme group, leading to the binding of four oxygen molecules to a single hemoglobin protein. Once oxygen is attached to hemoglobin, it is renamed oxyhemoglobin. Red blood cells then carry oxyhemoglobin throughout the body, delivering oxygen to tissues in need. Oxygen is released from the heme groups when hemoglobin is exposed to slightly acidic blood, a condition that arises when there are high concentrations of carbon dioxide in the blood. Gaseous carbon dioxide reacts with water to form carbonic acid, causing a slight increase in blood acidity. High levels of carbon dioxide in the blood are often associated with increased metabolism, such as during exercise or periods of stress. The more carbon dioxide is generated, the more oxygen is released into the bloodstream, a process that generally works seamlessly.
However, the introduction of carbon monoxide disrupts this well-regulated system. Carbon monoxide binds to heme groups on hemoglobin, displacing oxygen molecules. This displacement allows only three oxygen molecules to bind to hemoglobin instead of the typical four. It's worth noting that when carbon monoxide binds to hemoglobin, the protein is transformed into carboxyhemoglobin, featuring different properties. Notably, carboxyhemoglobin imparts a bright cherry red color to the blood, a telltale sign of carbon monoxide poisoning. Individuals who have inhaled a significant amount of carbon monoxide exhibit a cherry red hue in their skin, a result of a substantial portion of their hemoglobin being converted to carboxyhemoglobin. Unfortunately, by the time the skin displays this distinct coloration, the victim is often already deceased, as high concentrations of carbon monoxide in the blood take the place of oxygen.
Displacing oxygen on hemoglobin is just one aspect of carbon monoxide's harmful impact. It indirectly affects other oxygen molecules as well. Carbon monoxide's charge stabilizes the iron ion at the center of the protein. This stabilization alters the physical structure of the heme groups, effectively trapping oxygen molecules in place as long as carbon monoxide is present. Consequently, the body cannot make use of the trapped oxygen. The inability to release oxygen into the bloodstream leads to asphyxiation, or oxygen deprivation.
The initial symptoms of asphyxiation typically manifest as flu-like symptoms, including headache, weakness, and dizziness. As more carbon monoxide is inhaled, symptoms progress to include chest pain, vomiting, confusion, irregular heartbeat, and loss of consciousness. Once unconsciousness is reached, the progression of symptoms is relentless, and the brain eventually shuts down, leading to fatality.
The time it takes for fatal effects to occur varies based on the concentration of carbon monoxide inhaled. At the lower end of the fatal concentration spectrum, around 3,200 parts per million, death can transpire within 30 minutes. At 12,800 parts per million, fatal effects may occur within three minutes or even less. In contrast, lethal concentrations of carbon dioxide are around 40,000 parts per million, but it is important to note that carbon dioxide is not inherently toxic. The harm it can cause is a result of its ability to displace oxygen and create an oxygen-deprived environment.
Another characteristic of carbon monoxide is its diffusive nature. It is less dense than both oxygen and carbon dioxide, allowing it to disperse efficiently in an enclosed space instead of settling near the ground. This quality adds to its insidious nature.
So, where does carbon monoxide originate? It is produced when combustion reactions occur in conditions with insufficient oxygen. In standard combustion reactions, hydrocarbons are burned in the presence of oxygen, leading to the production of water and carbon dioxide. However, when oxygen is scarce, these reactions proceed differently. Instead of producing carbon dioxide, only one oxygen atom binds to carbon, forming water and carbon monoxide. Such low-oxygen combustion reactions frequently occur when something is burning in an enclosed space with limited oxygen availability. One common scenario is an internal combustion engine running in a closed area like a garage. Carbon monoxide can also be generated within homes when furnace ventilation becomes blocked, a common occurrence in the winter. Additionally, burning charcoal can release significant amounts of carbon monoxide during combustion. This aspect was notably used as a means of suicide by K-pop singer Chung Young.
In everyday life, millions of individuals engage in activities that expose them to carbon monoxide. Smoking, whether it involves burning tobacco or cannabis, leads to direct inhalation of carbon monoxide. The combustion of these materials does not occur with adequate oxygen, resulting in carbon monoxide production. As a consequence, smoking introduces carbon monoxide directly into the bloodstream. Regardless of whether these substances are rolled in paper or smoked from a pipe, the combustion process remains oxygen-deficient. The consequences are higher levels of carbon monoxide in the blood.
A study published in a Spanish clinical journal tested 228 smokers for the presence of carbon monoxide in their blood. Non-smokers typically exhibit a one percent carbon monoxide concentration in their blood, primarily originating from biological processes and minor concentrations in the air. In contrast, smokers who consumed between one and ten cigarettes per day displayed a blood carbon monoxide concentration of 5.2 percent. For individuals who smoked over 20 cigarettes per day, the concentration reached 9.6 percent. Blood levels of 10 percent or higher are defined as carbon monoxide poisoning. Many of the symptoms experienced by chronic smokers, such as fatigue, headaches, shortness of breath, and confusion, are likely the result of mild carbon monoxide poisoning. It is worth noting that chronic smokers have more hemoglobin in their blood compared to non-smokers, as their bodies need to compensate for the inactivation of hemoglobin by carbon monoxide.
Although carbon monoxide poisoning is a highly dangerous condition, prompt recognition and treatment can be effective. Patients are typically administered oxygen via breathing masks, and, if necessary, placed in hyperbaric oxygen chambers. Breathing nearly pure oxygen in these chambers helps carbon monoxide molecules unbind from hemoglobin more rapidly, reducing the attachment time from five hours to approximately an hour and a half.
About the Creator
Ananymus Kelly
meticulous




Comments
There are no comments for this story
Be the first to respond and start the conversation.