Blood-brain Barrier Permeability
Blood-brain Barrier Permeability
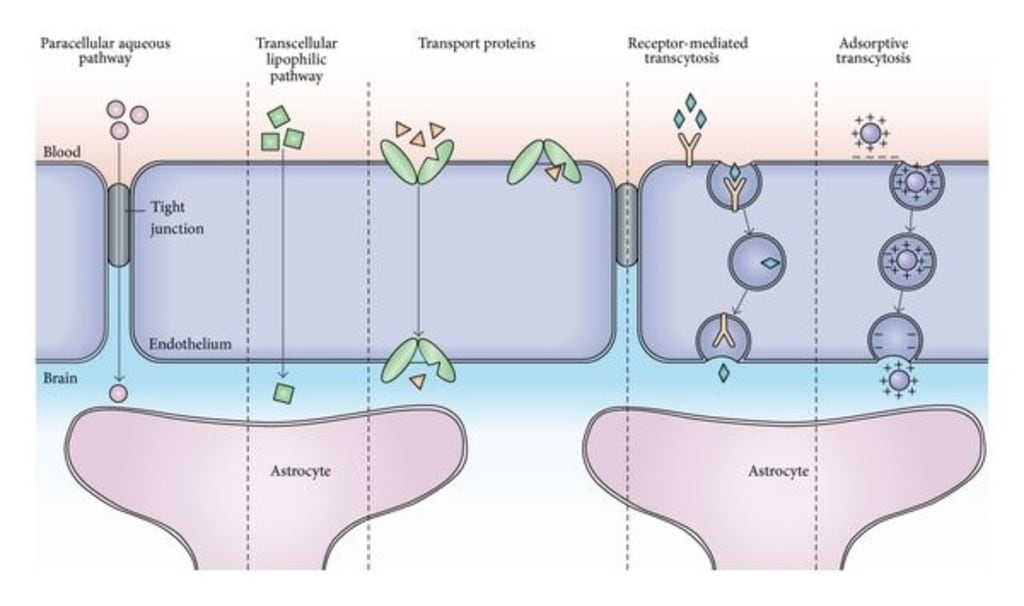
The blood-brain barrier is a barrier system in which the capillary endothelial cells in the brain are closely connected to each other while interacting with surrounding pericytes and astrocytes. It precisely controls the exchange of substances between blood and brain tissue, which is essential for maintaining the stability of the microenvironment in the brain. Studies show that the cells that make up the blood-brain barrier regulate the development and function of the blood-brain barrier by expressing tight and adherent connexins, transporters, and related signaling molecules. In addition, neurons and microglia are also involved in the regulation of the blood-brain barrier under physiological and pathological conditions. Recent studies have shown that the occurrence and development of various neurological diseases are accompanied by the destruction of the structure and function of the blood-brain barrier. Therefore, the study of the blood-brain barrier will deepen the understanding of neuro-vascular interactions and provides an important theoretical basis for the diagnosis and treatment of neurological diseases.
Blood-brain Barrier Permeability Function
As early as the early 20th century, Paul Ehrlich and Edwin Goldmann discovered the existence of a physical barrier between the central nervous system (CNS) and the peripheral blood circulation system. Subsequent studies have found that vascular endothelial cells in the brain are closely linked to each other through various connexins and interact with pericytes and astrocytes to form a special barrier system of the blood-brain barrier (BBB). In the process of maintaining energy supply of the brain and the stability of the microenvironment, BBB permeability plays a huge role in the normal function of the nervous system. For example, BBB permeability strictly limits the entry of neurotoxic substances, inflammatory factors, immune cells, etc. into the CNS, and excretes metabolites and neurotoxic substances in the CNS. Through precise control of blood and brain exchange, BBB maintains ion balance, water balance, and neurotransmitters and hormone levels in the CNS, thereby maintaining the homeostasis of the brain's microenvironment and ensuring proper functioning of the nervous system. Numerous studies have shown that the development and function of BBB are coordinated by vascular endothelial cells and pericytes, astrocytes, microglia, and neurons. BBB's development and dysfunction disrupt the homeostasis of the brain's microenvironment, leading to neuronal cell death and neurological dysfunction. In clinical studies, BBB dysfunction is found in many diseases of the nervous system such as stroke, Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). The low permeability of the BBB is also a barrier for neuro-therapeutic drugs. Therefore, drug delivery across the blood-brain barrier is also one of the research hotspots. To provide an important theoretical basis for the diagnosis and treatment of neurological diseases, an in-depth study of BBB permeability is essential.
Blood-brain Barrier Permeability Research Status
In the early stage of cerebral vascular development, the perivascular vascular plexus grows into the brain through angiogenesis under the induction of VEGF. Early developmental cerebral blood vessels have shown many of the characteristics of BBB, including tight and adherent expression of connexins and nutrient transporters, but there are still many endocytic transport vesicles and leukocyte adhesion molecules to regulate the endocytic transport. With the recruitment of pericytes and astrocytes, tight and adherent connexin localization is gradually accurate, endocytic transport is reduced, leukocyte adhesion molecule expression is down-regulated, outward transporter expression is increased, and BBB is gradually matured. In the process, many factors are involved in the development and maturation of BBB, such as VEGF, Wnt, Sonic Hedgehog (Shh), G protein-coupled receptor 124 (Gpr124), and the main promoter superfamily 2a.
VEGF is a critical factor in the development of vascular and lymphatic systems, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor. VEGF mainly passes through three tyrosine kinase receptors, VEGFR1 and VEGFR2, VEGFR3 and co-receptors neuropilins (NRPs) play a role. Vegfr2-deficient mice develop systemic vascular dysplasia and eventually die around 9 days in the embryo. Similarly, homozygous or heterozygous deletions of VEGF can lead to early embryonic death. During embryonic development, neural precursor cells located in the subventricular zone secrete VEGF, which guides the growth of new blood vessels into the brain. Deletion of VEGF in nerve cells leads to decreased vascular density and vascular dysplasia in the cerebral cortex and retina, affecting the establishment and function of BBB.
In neural cells, Wnt binds to the Frizzled receptor on vascular endothelial cells, inhibits the degradation of β-catenin, promotes the accumulation of β-catenin in the cytoplasm into the nucleus and increases the expression of β-catenin to induce Dr6 and Troy to promote angiogenesis and BBB formation in the brain. Dr6 and Troy can interact with downstream molecules of VEGF to regulate brain blood vessels and BBB development. In addition, studies have shown that neural progenitor cells in the forebrain and ventricle of mice express Wnt7a and Wnt7b, and neural precursor cells in the hindbrain and spinal dorsal cells express Wnt1, Wnt3a, and Wnt3b. The Wnt/β-catenin signaling pathway in CNS vascular endothelial cells is specifically activated during mouse embryonic development. Mice knocked out of Wnt7b developed severe cerebral hemorrhage and ventral vascular dysplasia in the brain, resulting in embryos death from E11.5 to E12.5. In addition, the absence of β-catenin in vascular endothelial cells specifically leads to abnormal vascular development in the CNS but does not affect the development of peripheral blood vessels. Studies find that Wnt signaling pathway is also capable of inducing expression of key BBB genes such as glut1.
In addition to the Wnt signaling pathway, Gpr124 also plays an important role in the formation of the BBB. Gpr124 belongs to the orphan receptor in the G protein-coupled receptor family and is highly expressed in CNS vascular endothelial cells, specifically mediating brain angiogenesis and BBB development. In mice, knockout of Gpr124 severely affects the survival, growth, and migration into the brain of CNS vascular endothelial cells, resulting in abnormal vascular development in the brain with ventral hemorrhage in the forebrain and spinal cord. Eventually, the embryo is killed. The phenotype of Gpr124 knockout mice is very similar to that of Wnt7b knockout or vascular endothelial cell β-catenin knockout mice, both of which are characterized by blood leakage and decreased Glut1 expression, suggesting that these two signaling pathways may be involved in BBB development. Recent studies have shown that Gpr124 acts as a coactivator of Wnt7a and Wnt7b. In addition, Gpr124 can cooperate with Norrin/Frizzled4 to regulate the integrity of CNS angiogenesis and BBB.
The Shh signaling pathway plays an important role in the formation and maintenance of BBB. Shh knockdown resulted in a significant down-regulation of tight junction proteins such as Occludin and Claudin-5, while the number of blood vessels in the brain was normal. Specific knockdown of Shh's downstream gene, vascular endothelial cells also leads to down-regulation of tight junction proteins and leads to leakage of plasma proteins from blood vessels in the brain. Unlike the Wnt signaling pathway, the Shh signaling pathway does not regulate angiogenesis and angiogenesis in CNS but significantly regulates BBB formation and integrity. The development and function of BBB are coordinated by a neurovascular network composed of pericytes, astrocytes, microglia, and neurons. Current studies show that neural progenitor cells are mainly involved in the induction of BBB characteristics in early development; then, pericytes and astrocytes are involved in the differentiation and maintenance of BBB characteristics. Deletion of pericytes leads to a significant increase in BBB leakage, endocytic transport, and increased expression of leukocyte adhesion molecules. By comparing the transcription of CNS vascular endothelial cells in Pdgfrβ-deficient and wild-type mice, it was found that the connexin and transporter-related genes are involved in BBB formation and maintenance. The expression of related proteins, such as PLVAP and leukocyte adhesion molecules, is significantly increased.
Relationship with Disease
AD
AD is a progressive neurodegenerative disease with progressive memory decline and cognitive impairment as the main clinical symptoms. Its main pathological feature is the deposition of neurotoxic β-amyloid in the brain. Studies have shown a significant increase in Aβ levels in the brain of familial and sporadic AD patients, but no increase in Aβ production, suggesting that Aβ clearance abnormalities lead to Aβ deposition in the brain. There are multiple transporters on the BBB involved in the clearance of Aβ. During the development of AD, abnormalities in BBB clearance of Aβ lead to a series of changes in neurovascular units and ultimately to the destruction of BBB.
Amyotrophic lateral sclerosis
BBB also plays a role in the development of ALS. Studies have shown that plasma components such as albumin, IgG, and complement are significantly elevated in the cerebrospinal fluid and spinal cord of patients with ALS, suggesting that ALS is associated with a blood-brain barrier and BBB destruction. Berislav V. Zlokovic proposed the Zlokovic-Cleveland model for studying the role of BBB in ALS. This model suggests that disruption of BBB can cause plasma proteins to leak into the spinal cord, causing hypoxia and edema in local tissues. Leaked IgG can directly bind to the surface antigen of neurons, and produce ROS and initiate autoimmune reactions, leading to demyelination, disruption of neurotransmission and neuronal death. Leaking red blood cells release hemoglobin, which directly causes ROS production, lipid superoxide, and neuronal death.
References:
- Olszewski J. The Blood-Brain Barrier. Lancet. 1960, 318(8246):584-585.
- Alluri H, Wiggins-Dohlvik K, Davis M L, et al. Blood-brain barrier dysfunction following traumatic brain injury. Metabolic Brain Disease. 2015, 30(5):1093-1104.
- Weiss N, Miller F, Cazaubon S, et al. The blood-brain barrier in brain homeostasis and neurological diseases. BBA – Biomembranes. 2009, 1788(4):842-857.
- András I E, Toborek M. Extracellular vesicles of the blood-brain barrier. Tissue Barriers. 2016, 4(1)
- Serlin Y, Shelef I, Knyazer B, et al. Anatomy and physiology of the blood-brain barrie. Seminars in Cell & Developmental Biology. 2015, 38(9): 2-6.
About the Creator
Creative Diagnostics
Creative Diagnostics is a leading manufacturer and supplier of antibodies, viral antigens, innovative diagnostic components, and critical assay reagents.





Comments
Creative Diagnostics is not accepting comments at the moment
Want to show your support? Send them a one-off tip.