The history of the universe is a history of constant change. However, while its state and appearance has changed drastically over the course of its 13.82 billion years of expansion, certain key things have remained unchanged. For one, the laws of nature have stayed constant throughout time and space, for every cubic planck-length of the universe.
We are very glad that everything in existence has agreed to play by the same rules, as that is the only way we are able to use the regularities of nature to conduct scientific experiments and describe the cosmos mathematically. By understanding those laws, I and many scientists have been able to piece together the history of the universe, the history of humanity.
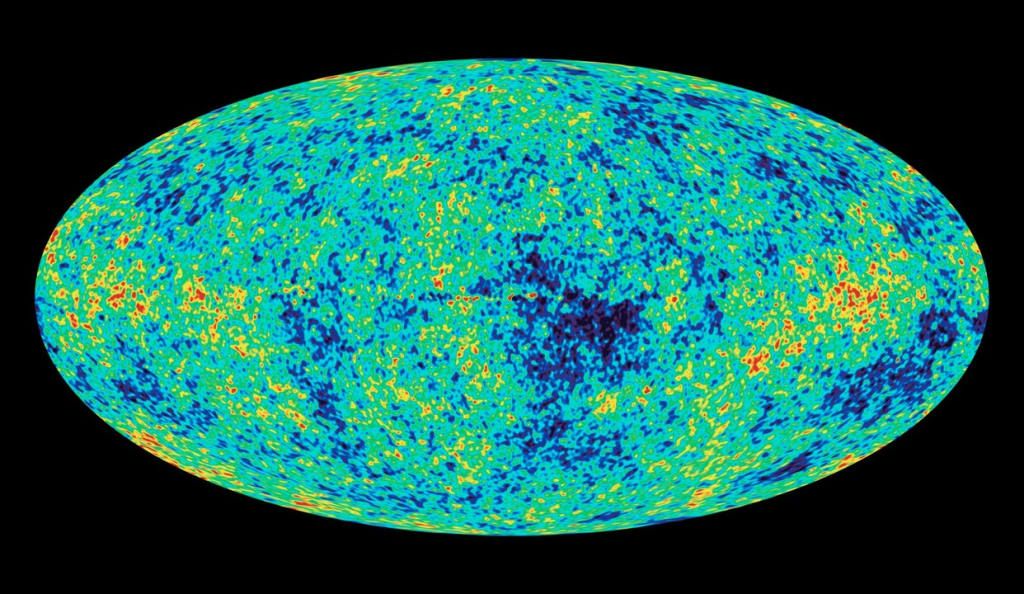
Another key feature of the universe that has been seminal to astronomical research is the fact that is so uniform. In technical terms, we refer to these properties as homogeneous and isotropic. Homogeneous, which means that every subatomic piece of the universe is identical to every other subatomic piece. And isotropic, referring to how matter and energy in the universe are evenly distributed.
It is so evenly distributed, in fact, that the microwave background radiation that makes up the perimeter of the visible universe is identical to one-one hundredth of a degree. Were it not for random fluctuations on the quantum level, there might not have been any way for structures like stars and galaxies to form at all.
One of the most fascinating consequences of these properties is that the entire history of the universe can be traced back from every single atom. Take for example a proton inside of one of the oxygen atoms that make up the water in your body.
That proton was first forged during the first few minutes of the universe, when quarks were no longer able to stay free. That proton, with its positive charge and tightly bound energy, then spent thousands of years zipping around, bumping too-and-fro between other protons exactly like itself, along with light, electrons, and a cornucopia of other particles.
When atoms first formed three hundred thousand years after the big bang, the universe became cold and dark. That proton then spent hundreds of thousands of years with nothing but a single electron orbiting around it. That was of course until trillions upon trillions of similar such hydrogen came together again due to their mutual gravity, until eventually they lost their electrons once again, and grew so close together that they actually began to fuse.
Some of the protons lost their charge and became neutrons, and together they clumped together to form heavier and heavier elements within the first stars. Among the largest of these stars, which only lived for hundreds of millions of years, the heaviest elements on the periodic table can be formed once iron is fused within their cores.
At that point, the stars collapse, and their outer layers ricochet out into space with more energy than a Sun-like star could put out in its entire lifetime. In that moment, that proton became bundled up into the nucleus of an oxygen atom, sharing quark-antiquark pairs with other protons and neutrons. It then wound up becoming part of a nebula.
Eventually, it bound itself up with hydrogen that never got fused, and formed into water, which bundled with other such molecules into ice boulders known as comets. Eventually, those comets took part in the great bombardment that goes into forging planets. That proton found its way to Earth's oceans, where it spent the subsequent four and a half billion years in rivers, clouds, streams, all varieties of plants and animals, and eventually into a faucet, into a cup, down your throat, and into each of your cells, until eventually it will pass through and continue its journey throughout time and space.
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments
There are no comments for this story
Be the first to respond and start the conversation.