Magic Mushroom Growing Tips
An expert in the cultivation of Stropharia cubensis shares his magic mushroom growing tips.

Stropharia cubensis can be found in appropriate habitats throughout the Southern US, all through the coastal regions of Mexico, and throughout coastal and equatorial regions of South America. In the US, it has been reported from Texas, Louisiana, Alabama, Mississippi, Arkansas, Florida, Tennessee, and Georgia. Its distribution would probably be even greater were it not for the fact that its environmental requirements limit it to regions of mild temperatures and high humidity.
Because of its specific habitat and singular appearance, Stropharia cubensis is one of the easiest mushrooms to identify. It can be found growing out of cow-pies in pastures during rainy warm seasons. Other dung-growing mushrooms may also be found in the same pasture, but these bear little resemblance to Stropharia.
Identifying Your Mushrooms

Image via WallpapersCraft
The following botanical description of Stropharia cubensis is taken from Mushrooms of North America by Orson K. Miller, Jr.
"Cap pale yellowish, persistent ring; blue-staining stalk."
"Cap 1.5-8 cm. broad, conic, bell-shaped, convex in age, viscid, without hairs, whitish to pale yellow, light brownish in age, stains bluish in age. Flesh firm, white, bruises blue. Gills adnate (attached) to adnexed (notched), close, grey to violet-grey in age with white edges. Stalk 4-15 cm long, 4-14 mm thick, enlarging somewhat toward the base, dry, without hairs, white staining blue when bruised. Veil white, leaving a superior membranous ring. Spores elliptical to oval in side-view, thick-walled, with a large pore at apex, purple-brown spore print. Cystidia (sterile cells) on gill edge club-shaped with rounded heads. Miller places this species in the genus Psilocybe, after Singer."
"The flesh of this mushroom exhibits the property of staining a bluish color when bruised or broken. This blue-staining reaction is apparently an enzymatic oxidation of some indole substrate (tryptophane, 5– dydroxzytryptamine, or psilocybin) and is a fairly reliable indicator of the presence of psilocybin, not only in Stropharia cubensis, but also in other closely related genera (members of the family Strophariaceae). Other mushrooms, such as members of the genus Russula, section Nigricantinae, and boletus, exhibit a similar blueing. The blueing in these cases, however, is not due to the presence of indole substrates and these mushrooms otherwise bear no resemblance whatever to Stropharia cubensis or related species."
Collecting Spores

Image via Wikipedia
Once one has located a specimen or specimens of Stropharia cubensis, and been satisfied as to its identity in all particulars, it is necessary to collect spores for cultivation. Spores can be easily collected in the following manner:
- Take one or more fresh specimens with the caps fully open.
- Using a sharp knife, cut off the stipe as close to the gills as is possible and then place the cap gillside down on a clean sheet of white paper, and leave for 24 hours. It doesn't hurt to cover the caps with a small bowl while taking the spore print in order to retard dessication.
- When the caps are removed, a dark-purplish, radially symmetrical deposit of spores will remain on the paper where the gills contacted it. The paper should then be folded and sealed in an envelope in order to prevent further contamination by air-borne spores of other species of lower fungi. A single spore-print contains tens of millions of spores, and is sufficient to make hundreds of spore germinations.
Once the spore-print has been collected, it is necessary to germinate some spores in order to begin the lifecycle that will eventually culminate in the production of more mushrooms.
All gilled fungi are members of the class Basidiomycetes, i.e., they are characterized by the production of spores on club-shaped appendages called basidia. Spores borne on basidia are called basidiospores. Most of the conspicuous fungi that one encounters, such as mushrooms, puffballs, and bracket fungi, are members of the subclass Homobasidiomycetes. Of the members of this subclass, the gilled mushrooms are placed in the order Agaricales. The basidiospores germinate to form a monokaryotic hypha. A hypha is a tubular filament; An aggregation of these hyphae collectively comprise a mass of thread-like filaments referred to as the mycelium. The mycelium comprises the main body, or thallus, of the fungus. The stalked, capped structure which we call the mushroom is actually only the "fruiting body" or the spore-producing reproductive structure and constitutes only a small portion of the total mass of the fungus, the great bulk of the organism exists underground in the form of a network of mycelium, which occasionally "fruits," or produces mushrooms, under appropriate conditions.
Compatible Mating Types
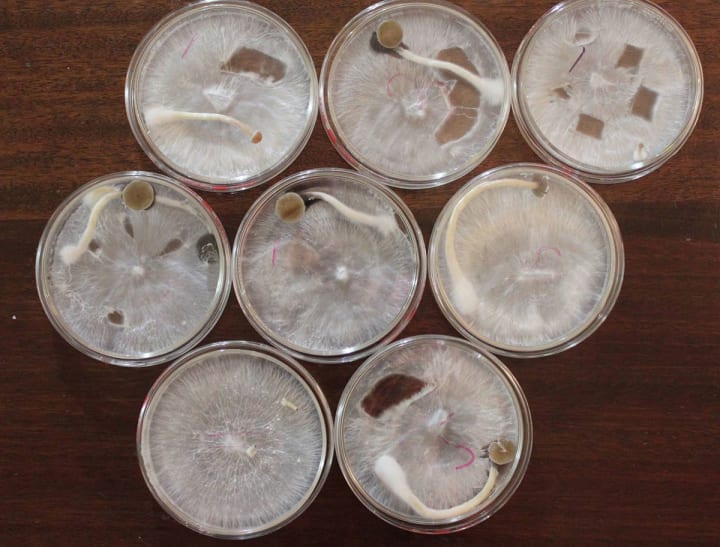
Image via Shroomery
The basidiospores germinate to produce a monokaryotic mycelium, i.e., a mycelium having only one nucleus per cell. This mycelium grows out until it encounters another monokaryotic mycelium, germinated from another spore, that is a compatible mating type. If the monokaryotic mycelium does not contact a compatible monokaryotic mycelium, it eventually dies. In situations where two compatible monokaryotic mycelia do make contact, however, a process called somatogamy, or a fusing of the somatic cells of the two mycelia, takes place, but fusion of the nuclei does not take place. The result of somatogamy is the establishment of a dikaryotic mycelium, i.e., a mycelium possessing two nuclei, one from each of the monokaryotic mycelia, in each of its cells.
The dikaryotic mycelium stage is the most prolonged portion of the life-cycle and is also the main assimilative stage of the fungus. The dikaryotic mycelium can propagate vegetatively indefinitely without going through a sexual (spore producing) stage. Given appropriate conditions, however, the dikaryotic mycelium can be induced to "fruit"; The undifferentiated mycelial thallus of the fungus begins to weave itself together into an artificial spore-bearing "fruiting body," in this case, into a mushroom. The mushroom continues to enlarge and thrust above the ground, incorporating more and more mycelium while at the same time expanding by absorption of water. At a certain stage in the growth of the mushroom, or basidiocarp, club-shaped structures called basidia form on the underside of the gills. At this point, karyogamy, or fusion of the two nuclei occurs within the basidia.
This is the only diploid, or second stage, for meiosis, or reduction division of a diploid (2n) nucleus to four haploid (n) nuclei occurs immediately following karyogamy. The result of meiosis is the production of four haploid nuclei within the basidium; These are then pushed out of the basidium and become surrounded by hard sheaths to form the basidiospores. The result is the basidium bearing four basidiospores on its outer surface. These basidiospores eventually detach from the basidium to begin the life-cycle again.
Fungi of the family Strophariacaea, which includes Stropharia cubensis and most other psilocybin-containing genera, are genetically complex with respect to the mating compatibility of different monokaryotic mycelia. These fungi are heterothallic and tetrapolar, that is, their sexual cycle is dependent on the fusion of two compatible monokaryotic mycelia, and their sexual compatibility is governed by two sets of factors:
In tetrapola heterothallism, two sets of factors, the A's and B.'s, are involved. If a sexually reproducing thallus is to be established, somatogamy must occur between mycelia differing in both sets of factors—for example AB x ab. The number of mating classes is somewhat greater than in bipolar forms, since four types typically arise from spores of a single basidiocarp. Obviously these mating types, numbering in the hundreds in both bipolar and tetrapolar species, cannot be designated as sexes!
Spore Germination

Image via Source of Spore
Keeping this information pertaining to the sexual characteristics of these fungi in mind, let us return to the problem of spore germination; The relevance of our digression into the matter of life-cycle and sexual compatibility will be seen shortly.
Once one has obtained a spore print from Stropharia cubensis, the monokaryotic mycelium can be easily obtained by germinating two spores on an appropriate solid nutrient medium, such as Potato Dextrose Agar or Malt Extract Agar.
For the present, however, simply assume that one has several clean, sterile petri plates which have been filled with an appropriate solid nutrient medium. Take the clean paper or slide on which the spore print is deposited, and using a clean knife, inoculating loop, or similar implement which has been sterilized by flaming in an alcohol lamp, simply scrape the spore print lightly with the implement, then transfer the adhering spores to the medium by touching the surface in one or more spots with the tip of the implement. Care should be taken to do this as quickly as possible, keeping the cover off the petri plate for the shortest time necessary, in order to minimize the chances for contaminating the plate with the airborne spores of contaminants.
A variation on this method can also be used: Instead of scraping the spores directly onto the plate, they can first be scraped into about 10 ml of sterilized water. Shake this vigorously, then dilute to 100 ml by adding sterile water. Using a sterilized pipette or syringe, take up 2-3 ml of diluted spore solution, and point-inoculate the petri plate by placing a drop of the solution at two or three separate points on the plate. Allow the covered inoculated plate to stand at room temperature for three to five days. During this time, the spores will germinate and monokaryotic mycelium will grow radially outward, from each point of inoculation. The plate should be left undisturbed until the mycelium from two different spores or two different points of inoculation has grown together and made contact. A few days after contact has occurred, one can be reasonably sure that somatogamy has taken place and that a dikaryotic mycelium has been established. Since in practice one scrapes off clumps of spores rather than single spores, onto the plate, waiting for two different inoculation spots to make contact is not really essential; By the time mycelial growth is well established on the plate, one can be confident of isolating dikaryotic mycelium.
Growing Magic Mushrooms

Image via Shroomery
In cultivating the fungus to the fruiting stage, one works primarily with a single strain of dikaryotic mycelium. However, because spores of several different mating types are produced by a single mushroom, a petri plate inoculated with spores will have possibly several dozen different strains of dikaryotic mycelium growing on it. It is therefore necessary to isolate one of the strains so that one can grow out stock inocula from a single, uniform strain. This can be accomplished using a scalpel, dissecting needle, or inoculating loop in which the loop has been bent to form a hook. The implement is first sterilized in an alcohol flame; then the petri plate is opened slightly and a very small piece of mycelial tissue is snagged on the end of the blade, needle, or hook, and transferred rapidly and deftly to a second clean sterile petri plate containing an appropriate solid nutrient medium. Dikaryotic mycelium is isolated using exactly the same techniques as are used in transferring mycelium from petri plates. By selecting a very small piece of tissue in this way, one can be reasonably sure that only one strain of dikaryotic mycelium is being removed and isolated. The dikaryotic mycelium thus isolated from the spore-germination plate will grow outward in all directions from the point of inoculation on the new plate and should cover most of the surface of the medium within eight to 12 days. One can then go ahead and make further transfers to new plates with a fair degree of certainty that one is working with a single strain of dikaryotic mycelium.
It is probably advisable to isolate several different strains of dikaryotic mycelium onto separate plates by taking tissue from different sections of the spore-germination plate. Different strains isolated from a single spore-germination plate should be identified by labels and compared for vigor of growth and vigor of fruiting ability, so that, through observation and trial-and-error, the strain showing maximum vigor in both respects can eventually be identified and used exclusively thereafter. Isolating the most vigorous strain takes time and careful observation. However, this need not interfere with continuing on to the second and third steps of the process, since any dikaryotic mycelium that has been properly isolated from other strains should exhibit fruiting ability. After several different strains have been put through several fruiting stages it should be apparent which strain is most vigorous.
If one has fresh mushroom specimens, it is possible to employ another method of isolating dikaryotic mycelium without utilizing spores. This is the method of subcutaneous isolation. Remove the stipe from a fresh mushroom, and, using three or four needles, fasten the cap gills down to a piece of cork-board or card-board. Swab the cap surface with tincture of iodine using a sterile cotton swab. Then, using a flame-sterilized scalpel, remove a small section of the outer cuticle of the cap. Then resterilize the scalpel, and remove a small piece of subcutaneous flesh from the cap, and transfer it to a sterilized nutrient plate. Since the flesh of the mushroom has been woven together out of dikaryotic mycelium, it will grow out across the plate in the same manner as mycelium isolated from a spore-germination plate. This procedure eliminates the step of having to isolate different dikaryotic strains, since mycelium isolated in this manner consists of only one strain. On the other hand, this procedure limits one to working with only one strain.
About the Creator
Ed Green
Gynecologist. Amateur Farmer and weed whacker. Loves figure skating and Liza Minnelli. Bakes amazing brownies.
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments
There are no comments for this story
Be the first to respond and start the conversation.