What Are Acids and Bases According to Arrhenius Theory?
Acids and base theory.
Introduction:-
In chemistry, the concept of acids and bases plays a fundamental role in understanding the behavior of substances. One of the earliest and most widely accepted theories explaining acids and bases is the Arrhenius theory. Proposed by Swedish chemist Svante Arrhenius in the late 19th century, this theory provides a foundation for comprehending the properties and reactions of these essential chemical entities. In this article I will aim to delve into What Are Acids and Bases According to Arrhenius Theory?and elucidate the characteristics, definitions, and applications of acids and bases.
Explanation:-
Acids According to Arrhenius Theory:
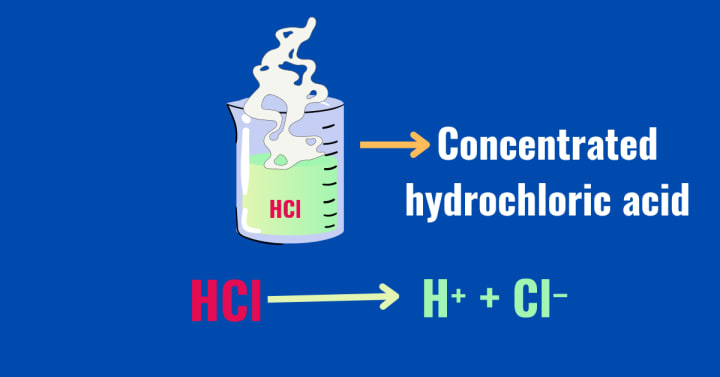
According to the Arrhenius theory, acids are the substances that, when dissolved in water, release hydrogen ions (H⁺). These hydrogen ions are the dominant species that are responsible for the characteristic properties of acids. An acid with a high concentration of hydrogen ions is considered a strong acid, while an acid with a low concentration of hydrogen ions is categorized as a weak acid.
Arrhenius also introduced the concept of dissociation to describe the behavior of acids in water. Acids dissociate, or break completely or partially, in water to form ions. For example, when hydrochloric acid (HCl) and HNO₃ dissolves in water, they completely dissociates to release hydrogen ions (H⁺), chloride ions (Cl⁻), H⁺, and No₃⁻ respectively.These dissociation reactions can be given as:-
HCl → H⁺ + Cl⁻
and
HNO₃→ H⁺ + NO₃⁻
The degree of dissociation determines the strength of the acid. Strong acids, like Hydrochloric acid, Nitric acid and Sulphuric acid completely dissociate in water, leading to a higher concentration of hydrogen ions. Weak acids, on the other hand, partially dissociate, resulting in a relatively lower concentration of hydrogen ions,like formic acid or methanoic acid(HCOOH) and acetic acid or ethanoic acid(CH₃COOH)
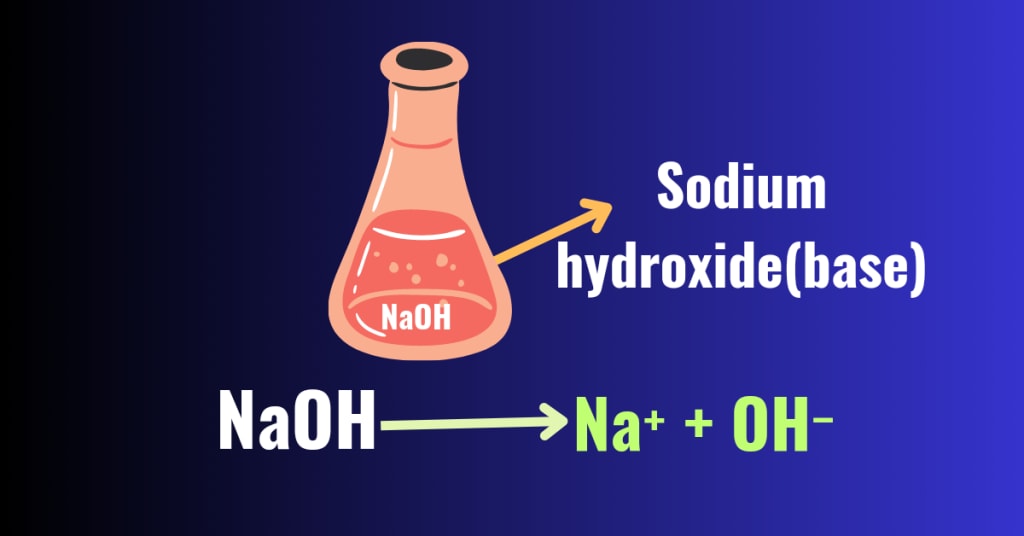
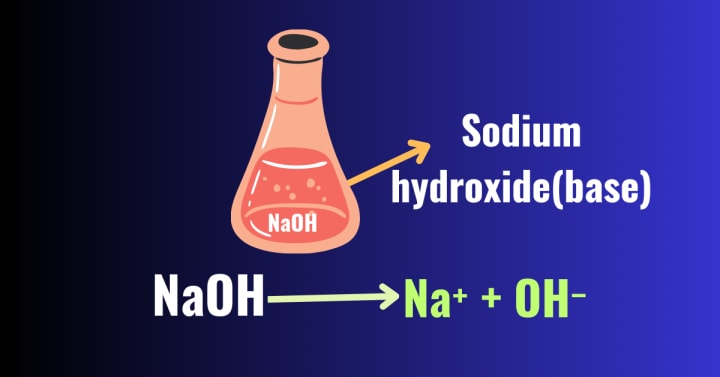
Bases According to Arrhenius Theory:-
According to the Arrhenius theory, bases are the substances that, when dissolved in water, release hydroxide ions or hydroxyl ions(OH⁻). These hydroxide ions are responsible for the characteristic properties of bases. Much like acids, bases are categorized as strong bases or weak bases based on the concentration of hydroxide ions they generate.
Arrhenius also introduced the concept of dissociation for bases. Bases dissociate completely or partially in water, forming hydroxide ions or hydroxyl ions(OH⁻). Bases which dissociates completely are called strong bases .Some examples of strong bases are sodium hydroxide (NaOH) potassium hydroxide (KOH), which dissociates in water completely as follows:-
Answer:- Hydrogen Potential of a solution can be defined as the strength of hydrogen ion (H⁺) concentration of a solution which is equal to the negative logarithm of hydrogen ion (H⁺).
Mathematically, it can be expressed as,
pH = - log [ H⁺]
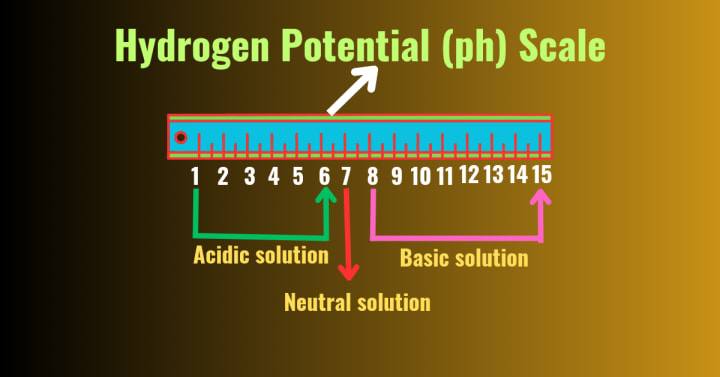
What is pH scale?
Answer:- A special scale which is used to identify the nature of a solution whether it is an acidic solution or basic solution or neutral solution.
A solution whose pH <7 is always an acidic,where as pH >7 is always a basic or base solution and pH = 7 is a neutral solution.
Applications of the Arrhenius Theory:
The Arrhenius theory of acids and bases has a large applications in various fields. For instance, it serves as the foundation for pH measurement, a crucial parameter in fields like medicine, environmental science, and wastewater treatment. The concentration of hydrogen ions in a solution determines the pH value, with low pH values indicating acidity and high pH values indicating alkalinity.
Mean while, the Arrhenius theory also gives insight into the corrosive properties of acids and the caustic nature of bases. Understanding the behavior of these substances helps prevent damage to materials, equipment, and infrastructure when handling corrosive or caustic solutions.
Conclusion:-
The Arrhenius theory, proposed by Svante Arrhenius that what are acids and bases?, provides a conceptual framework for comprehending the behavior, properties, and reactions of acids and bases. According to the theory, acids release hydrogen ions (H⁺) when dissolved in water, while bases dissociate completely or partially hydroxide ions (OH-). These concepts of dissociation are crucial in understanding the strengths of acids and bases.
Furthermore, the Arrhenius theory explains how acids and bases neutralize each other in chemical reactions. Understanding this neutralization process has practical applications in various fields, such as pH measurement and maintaining safety when working with corrosive or caustic substances.
While the Arrhenius theory represents an important step in understanding acids and bases, subsequent theories, such as the Bronsted-Lowry..........
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.



Comments
There are no comments for this story
Be the first to respond and start the conversation.