Understanding Hydrogen Bonding: A Special Intermolecular Interaction
Exploring the Intricacies and Importance of Hydrogen Bonding in Chemistry and Biology
While studying chemistry, you must have read about hydrogen bonding, a special type of dipole-dipole attraction. Technically, it's not a real bond in which electrons are shared between two atoms or transferred from one atom to another. Instead, it's an interaction similar to the Van der Waals force.
How Does Hydrogen Bonding Work?
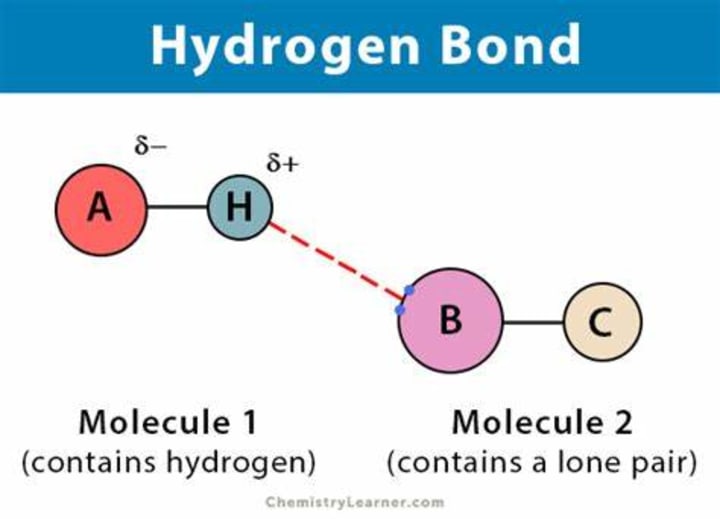
When a hydrogen atom is covalently bonded with an atom, let's say atom X, which is more electronegative than hydrogen, a partial positive charge on hydrogen and a partial negative charge on atom X would be generated.
This happens due to the difference in electronegativity between the two atoms. Electronegativity can be thought of as the ability of an atom to pull the shared pair of electrons. Because atom X has higher electronegativity, it pulls hydrogen's electrons towards itself.
Thus, the hydrogen atom acquires a positive charge due to the electronegative atom X. What if, in this situation, another electronegative atom (let's say atom Y) comes near hydrogen? Again, due to the difference in electronegativity, there would be an interaction between electrons of atom Y and the hydrogen atom; this interaction is known as hydrogen bonding.
Because the covalent bond is much stronger than the hydrogen bond, the length of the X-H bond would be smaller than the H-Y bond. It's because the bond strength is inversely proportional to the bond length.
A hydrogen bond is much weaker than ionic and covalent bonds but stronger than the Van der Waals force. The bond energy of Van der Waals forces and ionic/covalent bonds is 8 kJ/mol and more than 200 kJ/mol, respectively. The bond energy of the hydrogen bond is around 8 - 42 kJ/mol. Bond energy is the energy required to break any bond; it can be used as a parameter to show the strength of any bond.
When Does Hydrogen Bonding Occur?
We know that the structure of hydrogen bonds is like X-H...Y, where X is covalently bonded with hydrogen and Y makes a hydrogen bond. Not all compounds containing hydrogen atoms will show hydrogen bonding. For hydrogen bonding to occur, certain conditions must be met:
- The electronegativity of atom X should be significantly higher than that of hydrogen.
- The electronegativity of atom Y should be more than hydrogen.
- The size of atom Y should be small, and it should have a lone pair of electrons.
Generally, the atom Y, which makes the hydrogen bond, is nitrogen, oxygen, or fluorine. These three atoms fulfill the conditions for atom Y. But in organic chemistry, chlorine and carbon can also form hydrogen bonds under specific circumstances. You will encounter hydrogen bonds frequently in organic chemistry.
There is a relationship between the bond energy of the hydrogen bond and the electronegativity of atoms X and Y. Typically, the bond energy of hydrogen bonding increases with the electronegativity of both X and Y.
Special Case (HF2-)
We know that a hydrogen bond is weaker than a covalent bond. However, there are exceptional cases like the bifluoride ion (HF2-), where the strength of the hydrogen bond almost matches the strength of a covalent bond.
In HF2-, it becomes difficult to distinguish between hydrogen bonds and covalent bonds. The HF bond has a bond energy of around 200 kJ/mol on both sides.
Types of Hydrogen Bonding
Depending on whether the hydrogen bonding is taking place between two different molecules or between two atoms within the same molecule, hydrogen bonds have been categorized into two types:
Intermolecular Hydrogen Bonding
Here, the hydrogen bond forms between different molecules of the same or different compounds. It is like an inter-school competition, where students of one school compete with students of another school. Similarly, in intermolecular hydrogen bonding, hydrogen from one molecule interacts with any electronegative atom of another molecule.
A very common example of intermolecular hydrogen bonding is water. Two hydrogen atoms in an H2O molecule interact with two oxygen atoms of other H2O molecules through hydrogen bonding. Due to the hydrogen bond, each oxygen is connected to two hydrogen atoms, and two hydrogens from the same molecule are connected with two oxygen atoms of other molecules.
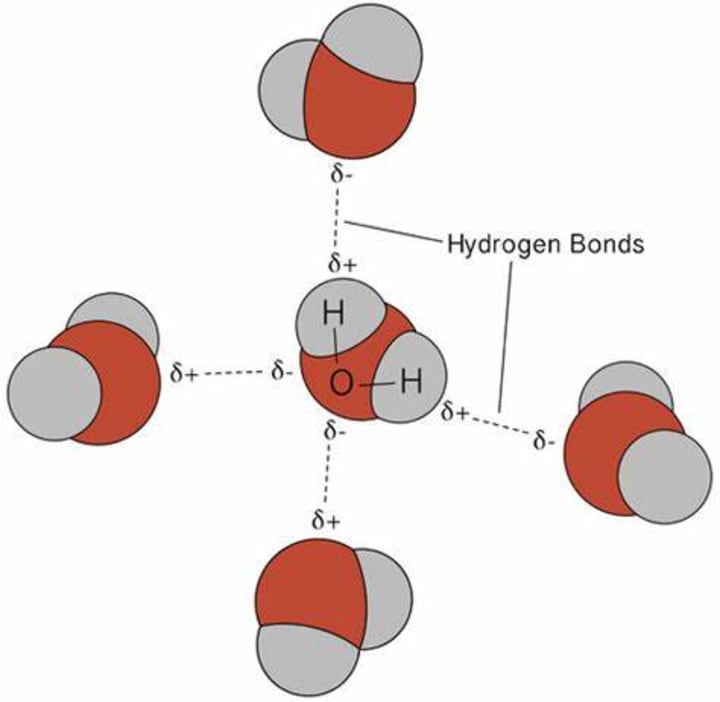
Do you know why ice floats on water? It's the hydrogen bonding that is responsible for this phenomenon. In ice, each molecule of H2O forms four hydrogen bonds and achieves a tetrahedral shape. However, due to its cage-like structure, it also creates voids (empty space). Due to these voids, ice becomes less dense than water, and thus it floats.
Another good example of intermolecular hydrogen bonding is boric acid. Theoretically, scientists concluded that boric acid must be found as H3BO3, but experimentally they found that it exists as (H3BO3)2; it's called a dimer (composed of two molecules).
Can you guess why it exists as a dimer? The answer is simple; it's due to hydrogen bonding. There are two hydrogen bonds in a dimer that keep two molecules together.
Generally, intermolecular hydrogen bonding decreases the strength of acids and increases properties like viscosity, boiling point, melting point, solubility, etc.
Intramolecular Hydrogen Bonding
In this case, a hydrogen bond forms within a single molecule. It's like an intra-school competition where students from the same school compete with one another. Intramolecular hydrogen bonding occurs when one functional group attached to a molecule has a hydrogen atom, and another functional group has electronegative atoms like nitrogen, oxygen, or fluorine.
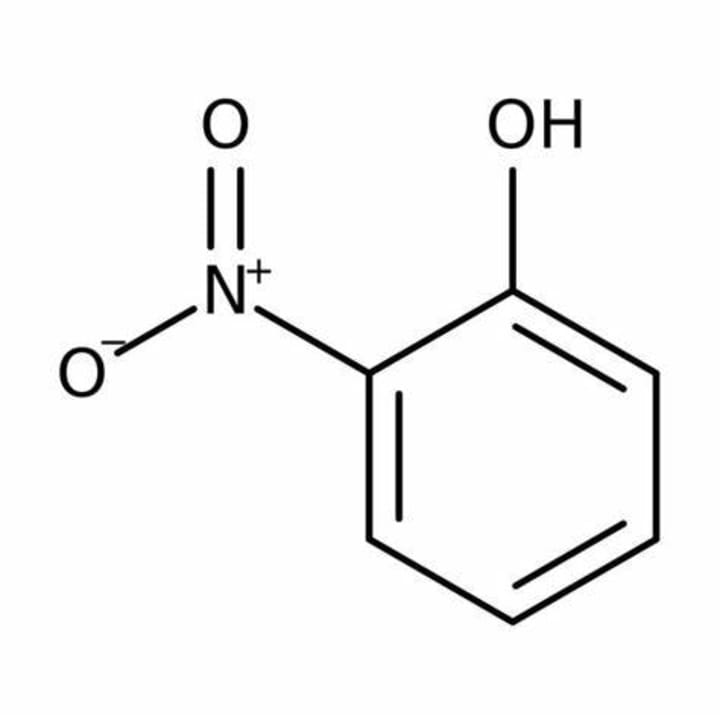
An example is ortho-nitrophenol, where a nitro group is attached to the ortho position of phenol (benzene ring). Hydrogen from the hydroxyl group interacts with oxygen from the nitro group and forms a hydrogen bond.
But what if we change the position of the nitro group to the para position? Will it form an intramolecular hydrogen bond?
In para-nitrophenol, where the nitro group is at the para position, it will form hydrogen bonds, but they will not be intramolecular. This is because, in the molecule, the nitro group is farther away from the hydroxyl group, making it easier to form hydrogen bonds with nearby molecules of para-nitrophenol.
If we compare intermolecular and intramolecular bonds, molecules with intermolecular hydrogen bonds have higher melting and boiling points. This is because intermolecular hydrogen bonding occurs between two different molecules, resisting the separation of these molecules and contributing to higher melting and boiling points.
Why is Hydrogen Bonding Important?

Hydrogen bonding is very important in chemistry and biology, playing an essential role in many chemical processes and natural phenomena.
The property of water that allows it to stick to itself is called cohesion, and the property that enables it to stick to other surfaces is known as adhesion. For example, when a droplet of water falls on a leaf, the hydrogen bond between water molecules (cohesion) is stronger than the hydrogen bond between water and the leaf (adhesion). This explains why water has high surface tension.
The primary structure of proteins is very simple. However, due to hydrogen bonding, proteins acquire complex structures like secondary, tertiary, and quaternary structures. Breaking the hydrogen bonds in these complex structures will revert the protein to its original primary structure.
Hydrogen bonds are also responsible for the double helix model of DNA, which consists of two intertwined strands. These strands are held together by base pairs that form hydrogen bonds with each other. The importance of this topic becomes evident when studying biological molecules and processes.
About the Creator
Roshan Kumar
A Random Blog Writer
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments
There are no comments for this story
Be the first to respond and start the conversation.