Why Does Ice Float On Water?
Ice is the solid form of water still it floats. But why?

Ice is the solid form of water, and generally, the solid form of any substance sinks into its liquid form. Solid aluminum, for example, will sink in liquid aluminum. But we all know ice floats on water; we've all seen it. But why is that?
Short Answer: The density of an object determines whether it will float or sink in water. If an object has a lower density than water, it will float; if it has a higher density, it will sink. Ice is about 9% less dense than water, which is why it floats. But why is ice less dense than water? And what are the factors that influence density? Let us explore.
Why Do Things Float in Water?

When we put any substance in water or another liquid, it experiences an upward force called the buoyant force. This force helps ice cubes float in your drink and icebergs float in the ocean.
The law of buoyancy, also known as Archimedes' principle, states that an object's buoyant force equals the weight of the water displaced by it. The buoyant force works upward against the gravitational force, which is why we feel lightweight while swimming in water.
Fun Fact: We are discussing Archimedes' principle right now. Did you know there is a fascinating story behind its discovery? Archimedes discovered this law while in a bathtub; he was so excited about his discovery that he leaped out of the bathtub and ran naked, shouting "Eureka!" I recommend reading more about the revolutionary Archimedes Eureka moment.
Recall your high school physics classes, where you learned that the weight of an object equals the object's mass multiplied by g (acceleration due to gravity). Similarly, the buoyant force equals the mass of displaced water multiplied by g.
We know that density is mass divided by volume; from this relation, we can say that mass is density × volume. Let's put this in the buoyant force equation. Now, we can rewrite the upward force as g x volume x density.
What Density Does an Object Need to Float?

Here, two forces are working on the object: the gravitational force pulling it down and the buoyant force pushing it upward. Both forces are working in opposite directions.
So, what do you think happens when an object floats on water? Using all the knowledge we have gained, we can now conclude that for floating, the buoyant force should be greater than the gravitational force.
But which factors determine whether these two forces are equal or not? Earlier, we rewrote the weight of water as D1 x g x V where D1 is the density of water (997 kg/m³) and V is volume. Similarly, we can also write the object's weight as D2 x g x V, where D2 is the object's density.
Because the volume of displaced water and the object is the same and g is constant, the difference between these two forces is only their densities. It's clear from the equation that the buoyant force will be greater than the gravitational pull if the density of the object is less than that of water.
In the case of ice and water, ice is less dense, and that's why it floats. But what if an object has the same density as water? Will it float or sink? It's most likely that it will float, but in that case, it will be fully immersed in water.
Why Is Ice Less Dense Than Water?

In the solid state of matter, molecules are tightly packed in a fixed repeating pattern known as the crystal lattice. In this situation, particles are not free to move. But when the substance is heated, the molecules start vibrating, breaking the lattice structure.
When a solid is heated, its particles gain energy and try to move freely. Eventually, they acquire the liquid state, where particles have more freedom to move. When matter is in this state, it has a lower density than its solid state.
If you keep heating it, the molecules will gain more energy, and the substance will take the gaseous form. When heated, solids become liquids, and liquids become gases, each time becoming less dense than before.
Similarly, if we do the opposite, lowering a substance's temperature, we will observe the opposite. A gaseous substance cools and transforms into a liquid, which then converts into a solid on further cooling. The substance becomes denser than before throughout this procedure.
We can conclude from the above that a substance is denser in its solid state than in its liquid phase. However, we know that water in liquid form is denser than its solid state (ice). The hydrogen bonding in water molecules is the reason for this special case.
Hydrogen Bonding in Water
The chemical formula of water is H₂O, where two hydrogen atoms are covalently bonded with oxygen. A covalent bond forms when two atoms share a pair of electrons. Because oxygen is more electronegative than hydrogen, it tries to pull hydrogen's electrons. The pair of electrons shifts to oxygen due to the difference in electronegativity. As a result, oxygen becomes negatively charged, while hydrogen becomes positively charged.
Because opposite charges attract, oxygen with a partial negative charge attracts hydrogen atoms from adjacent molecules. This type of interaction is referred to as hydrogen bonding.
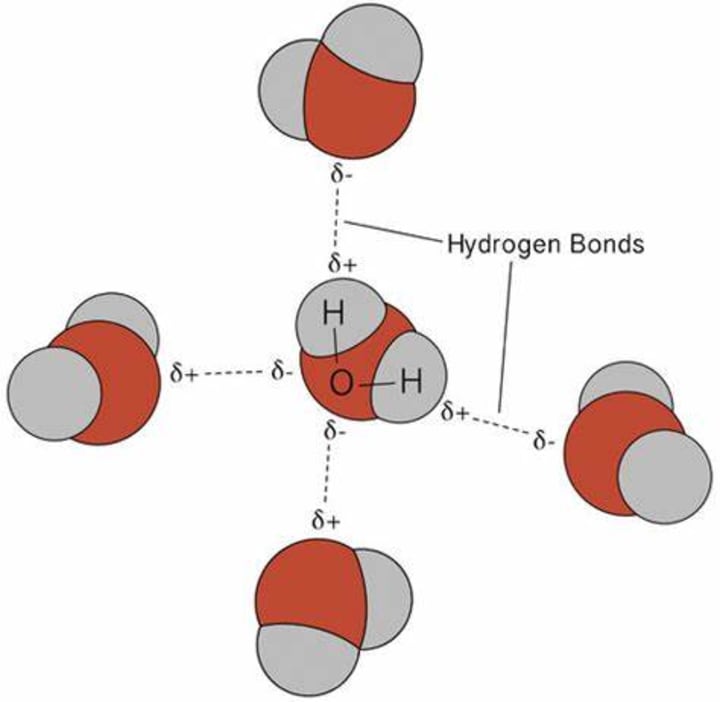
As shown in the image, water molecules form four hydrogen bonds and take on a tetrahedral shape. You can think of this as a cage-like structure. In freezing water, voids (empty spaces) are created, which increases the volume and decreases its density.
Ultimately, these voids are responsible for the floating ice. But do you know precisely at what temperature these voids start to form significantly? You might think it would be 0°C, but you are wrong. It's 4°C when the number of hydrogen bonds and voids increases significantly with decreasing temperature.
Water at 4°C
Ice is the organized structure of H₂O molecules with an excessive number of voids. Heat causes the hydrogen bonds in water to break, resulting in a denser, disordered structure of water.
A maximum number of hydrogen bonds are broken once the water reaches a temperature of 4°C. Water is densest at this temperature. Here's an interesting fact: at 4°C, the density of water will decrease whether you increase or decrease the temperature.
When the temperature is reduced, the number of hydrogen bonds increases, causing a decrease in density. If you increase the temperature, the density will still fall since no more hydrogen bonds can be broken; hence the heat will supply energy to molecules to move further apart.
However, the rate of decreasing density when water is heated at 4°C is much lower compared to when it is cooled. You might be astonished that water at 99°C is denser than water at 0°C; that's why ice floats even in hot water.
What Would Happen if Ice Were Denser Than Water?

For the sake of curiosity, suppose there is a parallel world where ice is denser than water. What are your thoughts on how that world would differ from ours?
Unfortunately, the Antarctic continent, the ice surface over the Arctic Ocean, and many living beings such as polar bears and penguins would not exist in a world where ice sinks into the water.
Water would freeze and sink to the bottom, making the ocean's bottom the coldest part. Many aquatic animals found at the ocean's depths would become extinct worldwide.
One of the reasons we are concerned about global warming is the melting of icebergs at the poles. The melting of ice raises the water level, putting many coastal cities at risk. So, in a world where ice is denser than water, many of our coastal cities, like Houston and Shanghai, would be underwater.
Aside from these, there would be many other differences, such as climate conditions, human lifestyle, and biodiversity. Can you think of any other distinctions? If so, please let us know in the comments section.
About the Creator
Roshan Kumar
A Random Blog Writer
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.






Comments
There are no comments for this story
Be the first to respond and start the conversation.