General Properties of Ionic Compounds
Ionic compound's property.

Introduction:-
Ionic compounds are a fundamental class of chemical compounds that play a vital role in various fields, including materials science, chemistry, and biology. They are formed through the strong electrostatic attraction between positively and negatively charged ions. In this article, I will try to explain into the general properties of ionic compounds, exploring their bonding nature, crystal structure, physical properties, and applications.
Explanation:-
To start this explanation of General Properties of Ionic Compounds let us first know,
What are Ionic compounds?
Answer:- The compounds which are formed by the transfer electrons from metal atom to non metal atom are known as ionic compounds. e,g; Potassium Chloride (Kcl), Aluminium oxide (Al₂O₃)
How are Ionic compounds formed ?
Answer:- In a chemical reaction, when a metallic atom gives up its valence shell electrons and a non metal atom accepts those electrons,then an ionic compound is formed.
Let,us discuss it more elaborately with one example:
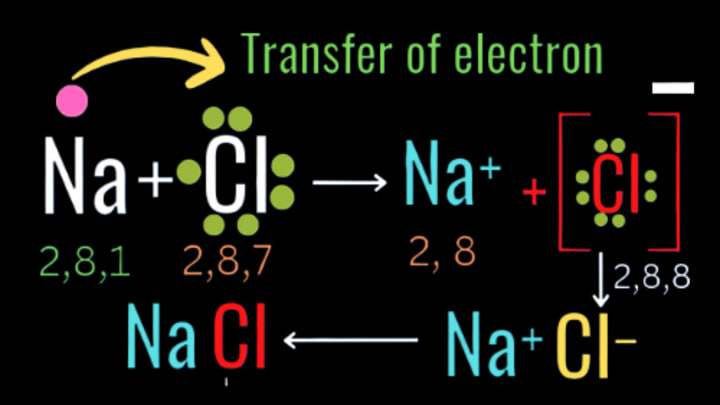
How sodium chloride is produced?
Answer:-
Sodium Chloride (Nacl): Na is a metal, the electronic configuration of sodium (Na) = 2,8, 1 to complete octet and become stable sodium needs to collect 7 electrons from other element to full fill its outer most shell i.e its third shell or 'M' shell to get its nearest noble gas Argon (2,8,8) and to full fil octet and become stable or Na has to give up its only valence shell electron (1) to get 2,8 electronic configuration of Neon as sodium is a metal so according to metals chemical property , all metals give up electrons they do not accept electrons or they are unable to accept electrons due to strong inter molecular force, thats why sodium is also unable to accept 7 electrons.
Again, chlorine (Cl) is a non metal and its electronic configuration is 2,8,7 so to fulfil its octet chlorine needs to accept one(1)electron or must have to give up its valence shell's 7 electrons to get either its nearest noble 2,8,8(Argon) or 2,8 (Neon) gas configuration to become stable due to non metals chemical property non metals never give up electrons, so chlorin is also unable to give up electrons.So in chemical reaction between sodium(Na) and chlorine ,Sodium (Na) gives up its lone valence electron to get its nearest noble gas Neon's configuration(2,8) and become stable in the mean time chlorine accepts the lone electron which is given up by sodium(Na) to get its nearest noble gas Argon's (2,8,8)configuration and become stable and by the process of electrons transfer they form ionic compound (Nacl)
The formation can be given by:-
Now I will start to explain General Properties of Ionic Compounds.
The General Properties of Ionic Compounds can be given as :-
(1)Ionic Bonding and Crystal Structure:-
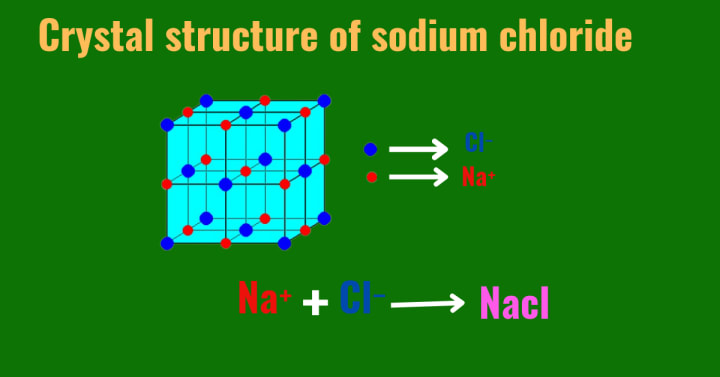
Ionic compounds are composed of positive ions, known as cations, and negative ions, known as anions. The bonding between these ions is predominantly electrostatic, resulting from the transfer of electrons from the metal (cation) to the non-metal (anion). This transfer leads to the formation of a lattice structure, where each cation is surrounded by anions and vice versa.e.g, Sodium Chloride (Nacl), Magnesium Chloride (Mgcl₂)
The crystal structure of ionic compounds is typically highly ordered and arranged in a repeating pattern. The arrangement is dictated by the size and charge of the ions. Common crystal structures include the face-centered cubic (FCC), body-centered cubic (BCC), and simple cubic (SC) structures.
(2)High Melting and Boiling Points:-
One of the significant characteristics of ionic compounds is their high melting and boiling points. This property is a consequence of the strong electrostatic forces of attraction between the ions within the crystal lattice. Significant amounts of energy are required to break these forces and convert the solid ionic compound into a liquid or gas.
(3)Solubility in Polar Solvents:-
Ionic compounds show varying solubilities in different solvents. However, they generally have high solubility in polar solvents, such as water, due to their ionic character. The polar character of water molecules allows them to surround and separate the individual ions, leading to the dissociation of the ionic compound into its constituent ions, and the process is known as dissolution or hydration.
(4)Electrical Conductivity:-
In the solid state, these compounds are typically poor conductors of electricity. However, when dissolved in water or molten, they become good conductors. This behavior arises from the presence of mobile ions in the aqueous or molten solution, which can carry electric charge. These mobile ions enable the flow of electric current, making ionic compounds essential in electrolytic processes and as electrolytes in batteries and fuel cells.
(5)Brittleness:-
Ionic compounds are often brittle solids. The brittleness stems from the strong electrostatic forces holding the ions in fixed positions within the crystal lattice. When an external force is applied, the ions of like charge are brought close to each other, leading to repulsion and subsequent fracture of the crystal lattice along specific planes. This brittleness explains why most ionic compounds cannot be deformed into wires or sheets without shattering.
(6)Color and Optical Properties:-
Many ionic compounds exhibit vibrant colors due to their ability to selectively absorb and transmit certain wavelengths of light. This absorption and transmission of light are associated with electronic transitions within the compound's structure. Transition metal ions, in particular, are renowned for their richly colored compounds, which find applications in pigments, dyes, and optical devices.
Applications of ionic compounds in Various Fields:-
Ionic compounds find widespread applications across different industries and scientific disciplines.
Some notable applications include:
a) Pharmaceuticals and Medicine: Many drugs and medicines are composed as ionic compounds to enhance their solubility, stability, and bioavailability.
b) Materials Science: Ionic compounds are integral to the design and fabrication of ceramics, glasses, catalysts, and superconductors.
c) Energy Storage: Ionic compounds acts as electrolytes in batteries and fuel cells, facilitating the conversion and storage of electrical energy.
d) Agriculture: Fertilizers often contain ionic compounds to provide essential nutrients to plants in an easily accessible form.
e) Chemical Analysis: Ionic compounds are carried out in analytical techniques such as ion chromatography and ion-selective electrodes to detect and ........




Comments
There are no comments for this story
Be the first to respond and start the conversation.