Carbohydrates
Introduction, properties and its classification.

Introduction:
A class of carbonyl compounds (aldehydes or ketones) that include many hydroxyl groups and are found in nature. On complete hydrolysis, several of their carbohydrate derivatives also form these types of molecules. Sugars and carbohydrates are the most numerous organic substances in nature, and they are also known as "saccharides." Some carbohydrates can be dissolved in water. Carbohydrates are made up entirely of carbon, hydrogen, and oxygen atoms, and their typical formula is Cn(H2O)n.
Carbohydrates' primary function is to:
Carbohydrates play a function in cell signaling, act as an energy source, and provide cells shape
The backbones of genetic biopolymers like ribonucleic acid and deoxyribonucleic acid are made up of chains of negatively charged phosphates that alternate with carbohydrates.
Carbohydrates account for 40–60% of the average caloric energy consumption in humans. The principal carbohydrates in the human diet include starches, sugar, and lactose. Starches and complex polysaccharides supply 60% of the digestible carbs in the usual adult Western diet, sucrose provides 30%, and lactose makes up 10% of the carbohydrates in the adult diet.
Carbohydrate classification:
Single sugars (monosaccharides) and polymers, oligosaccharides, and polysaccharides are examples of simple carbohydrates.
Monosaccharides
The most basic type of carbohydrate, sometimes referred to as simple sugars because they cannot be further hydrolyzed.
Colorless, crystalline solids that are water soluble but not non-polar solvent soluble.
A free aldehyde or ketone group is present in these compounds.
Cn(H2O)nor CnH2nOn is the general formula.
They are characterised by the number of carbon atoms they possess as well as the functional group they contain
Trioses, tetroses, pentoses, hexoses, heptoses, and other monosaccharides with 3, 4, 5, 6, 7,... carbons are known as trioses, tetroses, pentoses, hexoses, heptoses, and so on, as well as aldoses or ketoses, depending on whether they contain an aldehyde or ketone group.
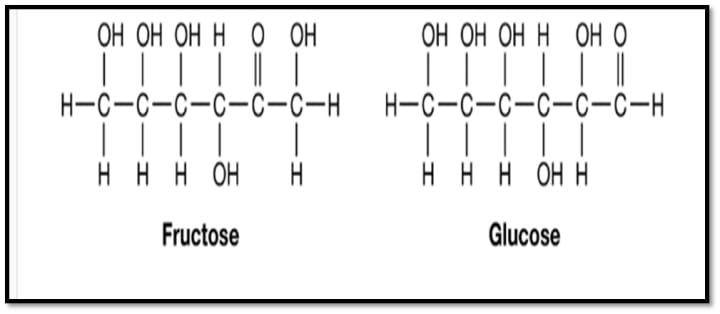
Glucose, Fructose, Erythrulose, and Ribulose are among examples.
Properties:
The majority of monosaccharides are sweet (fructose is sweetest; 73 percent sweeter than sucrose).
At room temperature, they are solids.
They have a high water solubility: Despite their high molecular weights, the monosaccharides are substantially more water soluble than most molecules of equivalent MW due to the huge amount of OH groups present.
Glucose can be made into a syrup by dissolving it in small volumes of water (1 g/1 ml H2O).
Oligosaccharides
Oligosaccharides are complex sugars that when hydrolyzed release 2 to 10 monosaccharide molecules of the same or different type.
Glycosidic linkage links the monosaccharide units together.
It's classed as a disaccharide, trisaccharide, tetrasaccharide, and so on, depending on the number of monosaccharide units. A disaccharide is an oligosaccharide that breaks down into two monosaccharides after hydrolysis.
Polysaccharides
They're also known as "glycans."
Polysaccharides are sugar molecules that contain more than 10 monosaccharide units and can be hundreds of sugar units long.
When hydrolyzed, they produce more than 10 monosaccharide molecules.
Polysaccharides differ from one another in terms of the identity of their recurrent monosaccharide units, chain length, bond connecting unit kinds, and branching degree.
They are essentially concerned with two key purposes: structural functionality and energy storage.
Polysaccharide classification:
They're further divided into groups based on the types of molecules created during hydrolysis.
1- Homopolysaccharidese:
Polysaccharides that contain monosaccharides of the same type.
Examples: starch, glycogen, cellulose, pectin.
2-Heteropolysaccharides:
Those polysaccharides containing monosaccharides of different types.
Examples: Hyaluronic acid, Chondroitin.
Function of Carbohydrates in Energy Formulation:
Carbohydrates have five basic functions in the human body.
Energy production in the body.
The ability to store energy
Constructing macromolecules.
Protein conservation.
Assisting in the metabolism of lipids.
Production of Energy
Carbohydrates' principal function is to provide energy to all of the body's cells. Many cells prefer glucose to other substances like fatty acids as a source of energy. Glucose is the only source of biological energy for some cells, such as red blood cells. About 70% of the glucose that enters the body through digestion is redistributed back into the bloodstream by the liver for utilisation by other tissues. The chemical connections between the carbon atoms provide energy to glucose. These associations are broken by the cells in our bodies.
Glycolysis is the initial step in the breakdown of glucose, and it involves a complex series of 10 enzyme reactions. The energy factory organelles, known as mitochondria, perform the second stage of glucose breakdown. More energy is obtained by removing one carbon atom and two oxygen atoms. The energy from these carbon bonds is transferred to another part of the mitochondria, allowing cells to use the cellular energy.
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.





Comments
There are no comments for this story
Be the first to respond and start the conversation.