Why Earth chose carbon-based life
What silicon-based life looks like
May I ask: Is there more carbon or silicon on Earth? If you answer that there is more silicon, congratulations, you are correct. There may be more silicon on Earth than you think. According to scientists' estimates, the total mass of carbon on Earth is only about one-thousandth of that of silicon.
So the question arises, if this is the case, why did the Earth choose carbon-based life instead of silicon-based life?
As we know, chemical reactions are the basis of life, so to figure this out, we need to start with the chemical properties of the elements.
As shown above, all the elements in the rightmost column of the periodic table are chemically very stable, and at room temperature and pressure, they are single-atom gases that are extremely difficult to react with, so they are called inert elements (or inert gases).
In terms of atomic structure, the number of electrons in the outermost layers of all six of these elements is eight, except for element 4, helium (helium has only two electrons), for example, element 10, neon (Ne) is such that
The number 18 element argon (Ar) is like this.
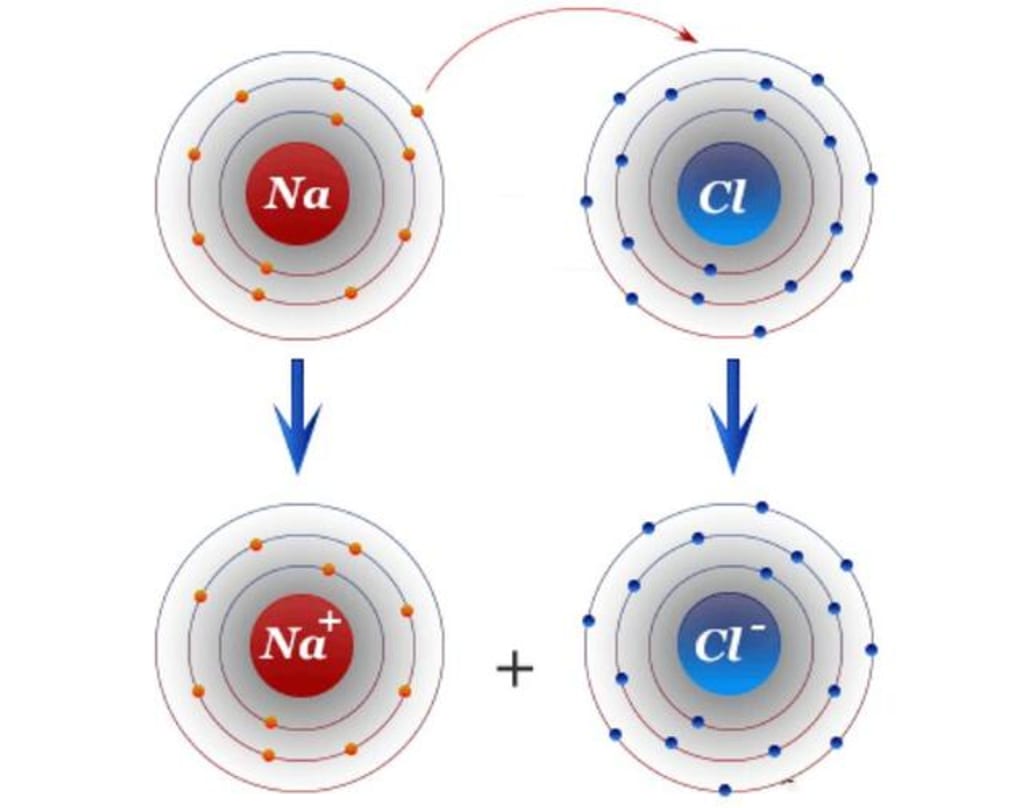
For each element, all tend to be "inherently" stable, so if given the opportunity, the non-inert elements in the periodic table will tend to form the same atomic structure as the inert elements, and if an atom has a small number of outermost electrons, it will tend to send away its outermost, For example, the atomic structure of element 11, sodium (Na), is as follows.
It can be seen that the sodium atom has only one electron in its outermost layer, and it only needs to send away this electron for its second layer to become the outermost layer, and with 8 electrons in this layer, it reaches stability.
Conversely, if an atom has a higher number of electrons in its outermost layer, it tends to get one electron from the outside world and its ability to get electrons is stronger, for example, the atomic structure of element 17, chlorine (Cl), is as follows.
As you can see, the chlorine atom has seven electrons in its outermost layer, and it only needs to gain one more electron to reach stability.
Because of this, both sodium and chlorine are very active chemically, and one of them wants to send away an electron every day, while the other one is always thinking about where to get an electron, and when these two meet together, of course, they hit it off, one happily sending and one happily taking.
After this, the sodium atom is positively charged because it lost an electron, while the chlorine atom is negatively charged because it gained an electron, so they are tightly bonded together to form a very stable sodium chloride molecule (NaCl, which is the main ingredient of table salt).
This combination of sodium and chlorine is called "ionic bonding". In addition to this "one sends, one takes" combination, there is another way to achieve stability between atoms, which is to share their electrons. For example, the number 8 element oxygen (O) looks like this.
As you can see, the oxygen atom has six electrons in its outermost layer, and it needs two more electrons to achieve stability.
For the sake of description, let's use anthropomorphism, i.e., for an oxygen atom, if there is another oxygen atom around it, it will negotiate with it: "Look, I am two electrons short, and you are also two electrons short, why don't we each take out two electrons to share, so that our remaining four electrons, plus the four shared electrons, will become a stable structure with 8 electrons?" The other oxygen atom thought about it for a while and thought that this was indeed the case, so the two oxygen atoms combined by sharing electrons to form an oxygen molecule (O2).
This type of bonding is called "covalent bonding". The chlorine atom mentioned earlier can also be bonded with another chlorine atom in this way to form chlorine gas, and it is worth noting that different types of elements can also be bonded by "covalent bonding", for example, an, For example, an oxygen atom can share electrons with two hydrogen atoms through a "covalent bond", so that the oxygen atom has 8 electrons in its outermost layer, and the atomic structure of both hydrogen atoms becomes the same as that of helium with two electrons, thus forming a stable dihydrogen monoxide (H2O), i.e. a water molecule.
With the above knowledge, let's look at the atomic structure of the element carbon (C).
Carbon is the number 6 element and has 4 electrons in its outermost layer. This is interesting because whether it is gaining or losing 4 electrons, the carbon atom can reach a stable state, and its ability to lose and gain electrons is the same. ".
This undoubtedly gives carbon atoms the ability to form complex compounds, for example, if a hydrogen atom needs to share one electron, the carbon atom will give it one, and if an oxygen atom needs to share two electrons, the carbon atom will give it two, and its remaining electrons can be shared with other carbon atoms, which can be combined with more atoms at the same time, and then through "covalent bonding " to another carbon atom ......
For the sake of understanding, let's compare the 4 outermost electrons of a carbon atom to 4 "hands". A large number of carbon atoms holding hands can form long chains, rings, nets, layers, and other complex structures. In these structures, almost every carbon atom has a "free" hand to pull other elements, thus greatly enhancing the complexity and diversity of compounds.
We know that organic compounds are the material basis of all life on Earth, and in fact, all these organic compounds are complex compounds with the carbon atoms "holding hands" as the "skeleton", and it is because of this that we consider all life on Earth (including ourselves) to be It is for this reason that we call all life on Earth (including ourselves) carbon-based life, which means that the so-called carbon-based life does not mean that the carbon content of living organisms is particularly high.
Okay, now let's look at the atomic structure of the element silicon (Si).
Silicon is the number 14 element, just below carbon in the periodic table, and it also has four electrons in its outermost layer, which means that silicon atoms have four "hands" like carbon atoms, and should also have the ability to form complex compounds.
This is the reason why there is such an expression as silicon-based life, which simply means that if all the basic substances that make up a certain kind of life are complex compounds with the silicon atoms "holding hands" as the "skeleton", then this kind of life is silicon-based life.
However, on Earth, silicon cannot form compounds with as much complexity and diversity as carbon, because the silicon atom has one more electron layer than the carbon atom, which makes its control over the four outermost electrons much lower than that of the carbon atom. This means that although the silicon atom also has four "hands", these "hands" are inherently weaker than those of the carbon atom.
For example, the compound methylsilane (SiH4, composed of one silicon atom and four hydrogen atoms), which is a compound of silicon and hydrogen, is subject to spontaneous combustion in the air, even at room temperature and pressure.
One important reason why carbon atoms can form long chains is that they can form double or even triple bonds, which we can simply understand as carbon atoms can reach out two or three hands to pull each other at the same time.
In the natural environment of the earth, silicon atoms are difficult to form double bonds, so the long chain structure formed by silicon atoms is very easy to break, so it is not possible to form complex compounds with the silicon atoms "holding hands" as the "skeleton".
In addition, the relatively strong bonding ability of oxygen and silicon leads to a strong tendency for the chemical reaction of silicon to produce silica and silicates and to form a structure called "silicon-oxygen tetrahedra" within these substances (as shown in the figure below).
In the Earth's natural environment, silica and silicates are very stable once they are produced and are extremely difficult to react with other substances, so although there is much more silicon than carbon on Earth, they are only present in large quantities in the form of inorganic substances, for example, many rocks on Earth (such as granite) are mainly composed of silicates, while the main component of our common sand is silicon dioxide.
It is for these reasons that the Earth has chosen carbon-based life over silicon-based life. Of course, the absence of silicon-based life on Earth does not mean that silicon-based life cannot exist in the universe; in theory, silicon-based life can exist in a given natural environment. If this is the case, what does silicon-based life look like? Let's continue to see.
Under high enough pressure, silicon atoms can form double bonds, and at low temperatures, compounds formed by silicon and non-oxygen elements can remain stable, but in addition, we have to exclude the combination of oxygen and silicon, so in a high-pressure, low-temperature and oxygen-deficient strong reducing environment, it is possible to form a "hand-in-hand" Thus, in a highly reducing environment with high pressure, low temperature and lack of oxygen, it is possible to form complex compounds with "hand-in-hand" silicon atoms as the "backbone" and thus evolve life.
Such conditions can occur on planets with low temperatures, where hydrogen, helium, nitrogen, methane, etc. can exist in liquid form, and if there are enough of these liquids, huge oceans can form, and at the bottom of these oceans, a high-pressure, low-temperature, oxygen-deficient, strongly reducing environment is formed.
However, since chemical reactions at low temperatures are usually not lively enough to support the activities of advanced life forms, even if silicon-based life exists in such conditions, they should be simple silicon-based microorganisms.
On the other hand, since oxygen and silicon have a strong bonding ability, if oxygen atoms are used to "build bridges", they can form "Si-O-Si-O-Si ......" A sufficiently high temperature allows the "SiO chain" to avoid the "SiO tetrahedron" structure described above, which in turn allows the "SiO chain" to appear as follows
This is equivalent to silicon atoms having a "free" hand to pull other elements, on top of which, if high pressure is added, compounds of sufficient complexity and diversity may be formed, providing the material basis for the evolution of life.
In the universe, if a planet with a rocky surface is massive enough, has a thick enough atmosphere, and is close enough to its host star, a high-temperature, high-pressure environment can exist on the surface of its planet, meaning that complex silicon-based life could exist on such a planet.
As early as the end of the 19th century, scientists suggested the possibility of silicon-based life in high-temperature environments, and in the days that followed, this speculation gained a certain degree of acceptance, according to people who envisioned that such silicon-based life would look like some kind of crystals, possessing a transparent or translucent appearance.
About the Creator
Robert Jack
One of the secrets of emotional stability for adults is to keep the expectations of others to a minimum.






Comments
There are no comments for this story
Be the first to respond and start the conversation.