Why Are Cities Unhealthy? Poor Biodiversity
New research shows how the outdoors makes us healthier – and how it can save our cities.

EVEN ON A BLUSTERY winter’s afternoon, Mount Lofty flaunts its splendour as a bushland oasis, one of the last vestiges of the original forests and woodlands that once dotted the Adelaide Plains of South Australia. As you meander down the narrow tracks from the summit, you feel invigorated by the scenery, the silence, and the smell of wet earth after a light shower. To a city dweller like me, the air itself seems therapeutic.
Which, as it turns out, is not an illusion.
Every time you walk through wild spaces that are replete with biodiversity and breathe the air, microbes that are gently wafting through the ecosystem land on your skin, enter your lungs, your gut, and become a part of you. There they join the billions already living in your ‘microbiome’ — the community of symbiotic microorganisms inside each human being.
Scientists have long known about these tiny symbiotic helpers in our bodies. But in the past decade or so, thanks to technological advances like molecular imaging, faster computers and rapid genetic sequencing, they have been astounded to realise just how crucial, and how widespread, is the role our microbiome plays in everything from nutrition to disease resistance — and even mental health.
The latest surprise is the discovery by researchers in Adelaide that the more diverse the microbes living in the soils around us, and the more you are exposed to them, the healthier you become. It’s a discovery that could change our cities, and make us healthier, on a global scale. And it all comes down to the humble earth.
THE HUMAN BODY is estimated to be made up of about 37 trillion individual cells — skin cells, liver cells, and so on. But the number of microbes living in, and on, our bodies — bacteria, archaea, viruses, protozoa and fungi — is thought to be more than double that number: about 100 trillion.
While we don’t give much thought to the microbes living inside us, they are actually essential to our survival, doing things that our bodies cannot. The microbiome is, in fact, an enormous invisible ecosystem of microbes that acts like a giant shadow organ, helping us to digest plant matter, regulate our immune systems, form new blood vessels, coordinate how our hormones behave, store fat and modulate brain signals. The bacteria therein also protect against other bacteria that cause disease, and produce vitamins including B vitamins B12, thiamine and riboflavin, and Vitamin K, which is needed for blood coagulation, or clotting.
Surprisingly, the microbiome was not generally recognised by scientists as a distinct part of the human body until the late 1990s. Since then, the understanding of their role has grown dramatically, to the point where the microbiome is now considered so crucial for living things that leading researchers have argued that animals and plants are not autonomous entities at all, but rather ‘holobionts’ — dynamic biomolecular networks of different species who work in a symbiotic relationship for mutual benefit.
Some 70% of the microbes in the human body are found in the gastrointestinal tract — the mouth, oesophagus, stomach and intestines that together make up a system of organs that takes in food, digests it to extract and absorb energy and nutrients, and expels the remainder as waste.
Study after study over the past 20 years has found that there’s a marked difference in the gut microbes of people who live in the countryside compared to those who live in cities: country dwellers have more diverse microbiomes; city folk have a lot less. A lack of microbe diversity is also found in people who suffer inflammatory diseases — like asthma and food allergies — which plague city dwellers, and whose incidence has soared in highly urbanised populations.

We now know that autoimmune diseases — like diabetes, rheumatoid arthritis, muscular dystrophy, multiple sclerosis, and fibromyalgia — are linked to dysfunctions of the microbiome. Because disease-causing microbes accumulate over time, they alter the genetic activity and the metabolic processes of the body, resulting in an abnormal immune response against substances normally present in the body. And these autoimmune diseases ca be passed on in families not by genetic inheritance, but by inheriting the family’s microbiome.
“There’s a whole host of benefits we derive from living in healthy environments,” said Philip Weinstein, a greying professor of biological sciences at the University of Adelaide with qualifications in both public health and ecology. “One of them is the ecosystem we’re exposed to — the rich diversity of microbes in the soil, in the air, in the food we eat and the animals we interact with.
“For most of our history as a species, we’ve had exposure to that, and that’s what we’ve evolved with and are adapted to live in — as well as eating high-fibre foods and running around all day,” said Weinstein, adjusting his metal-rimmed glasses. “But the movement of people into cities has basically cut us off from that environment. And all of those diseases of the urbanised Western lifestyle — like cardiovascular disease, diabetes, obesity, asthma — they have increased dramatically.”
Researchers now know the two are connected, Weinstein said. But the focus of health professionals has been on diet and exercise alone. Little attention has been paid to the role that ecosystems around us can play in improving our health — especially the health of our gut microbiome. And while we can’t reverse urbanisation and return to the savannas of Africa where humans evolved, we can bring those ecosystem benefits back to our cities.
That’s what Weinstein and Dr Martin Breed, of nearby Flinders University, are trying to do. The duo run the Healthy Urban Microbiome Initiative (HUMI), which has been exploring how adding microbial diversity to urban environments might improve the health of people by boosting the population of good bacteria in their microbiomes. And they’ve got the United Nations interested.

“There are more species of bacteria, fungi and other microbes out there than anything else — all this incredible and mostly unknown diversity, which perform all sorts of different functions for, not just us, but the natural world,” said Breed. “Plants form symbiotic relationships with many different microbial species, which provide the resources they need to grow. In healthy soil in the wild, in a quarter of a gram of soil, you often find 2,000 to 5,000 species — which is phenomenal.
“But when you look at degraded environments, like those in cities or crop pastures, there’s usually a very different suite of microbes, far lower in diversity. And often what’s there is an abundance of opportunistic species that can tolerate degraded environments, but are not all that good for plants, animals or for us.”
Breed is an affable young ecologist with a trim beard and ready smile. He began his scientific career restoring ecosystems of degraded land when, while working in Sweden, he came to appreciate how a range of new techniques in genomics — the interdisciplinary field of biology that explores the structure, function and evolution an organism’s complete DNA — might help explain why some restored ecosystems thrive, and some don’t.
He suspected that soil microbes played a part, but he needed evidence. Back in Australia, Breed and PhD student Craig Liddicoat and others collected soil samples from 200 sites across the country; some were from patches of degraded land, and some from wilderness areas rich in biodiversity. They then analysed the genes in the soil microbiomes to compare them. And found consistent patterns in the proportions of opportunistic versus stable bacteria.

Degraded landscapes were dominated by opportunistic ‘copiotrophic’ bacteria — such as Bacillus, Clostridium, Enterobacter, Legionella and Pseudomonas — which tend to thrive in nutrient-rich environments, like sewage lagoons, and are in the same genus as many disease-causing pathogenic bacteria in humans. In the more natural locations these were also present, but in much smaller numbers; more abundant were ‘oligotrophic’ species, which thrive in lower nutrient ecosystems and are involved in decomposition and nutrient recycling, like Bradyrhizobium, which helps plants extract nitrogen from the soil.
It was evolution at work: where humans were active and nutrients were plentiful, copiotrophs took over; in more pristine environments, the copiotrophs were out-competed by more efficient, and less energy needy, oligotrophs.
Could they restore the soil microbiome of degraded sites, and bring them back into a more natural balance by encouraging the ‘good’ bacteria and discouraging the ‘bad’? Breed’s team decided to find out: at various degraded sites, carefully selected plants were cultivated to encourage more diverse bacteria, and then monitored to see if changes occurred to the soil microbiome.
“There was dramatic change,” he said. “The microbial communities [in the degraded sites] — whether we looked at bacteria, fungi, microeukaryotes, even archaea — they were showing a trajectory of returning to a biodiverse state after just six to 10 years. This was a huge surprise, because we didn’t know microbe communities could return so rapidly.”
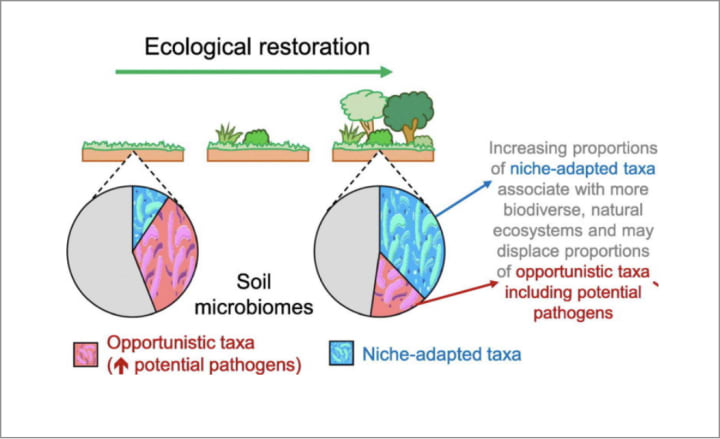

Breed calls the process ‘microbiome rewilding’, and it piqued the interest of Weinstein. The biodiversity of soil microbiomes outside cities might not only help explain why country folk tend to have healthier immune systems, but also offer a pathway to bringing those health gains to city dwellers.
“Among the benefits we’ve lost from our past is regular exposure to antigens, like those in microbes and allergens like pollen,” said Weinstein. “The diversity of earlier ecosystems trained our immune systems, but that training is no longer happening as much. Kids growing up on a farm are exposed to more pollens and microbes, and that trains their immune systems, so they do better. But city kids aren’t, so their immune systems are bored. So, when a pollen grain comes along, there’s an asthma attack, because their immune systems go into overload.”
It’s partly cultural, said Weinstein. Since 1864, when Louis Pasteur established modern germ theory — proving in the laboratory that microorganisms cause disease — we have been at war with microbes. Sanitation, personal hygiene, vaccines, pasteurisation and antibiotics have laid waste to a plethora of infectious diseases, and even drove some pathogens, like smallpox, to extinction.
This has been good for humanity. But we’ve gone overboard, Weinstein said: “The majority of urban populations now live in an environment that’s safe from those environmental pathogens like cholera and typhoid. But we’ve still got a cholera mindset, and that’s a mismatch.”
AT TRINITY GARDENS primary school in suburban Adelaide, an experiment is underway to see if introducing a more biodiverse plant ecosystem can improve the soil microbiome in an urban setting. In 2015, the school completed the conversion of two ovals into Portrush Forest — an outdoor learning environment with native vegetation, wetlands, vegetable gardens, an orchard and an assortment of nature walks scattered with fallen logs, cubby houses and sandpits.
The school grounds cover three suburban blocks, and Portrush Forest takes up a third of the northern-east corner. At 11 sites around the school, petri dishes are open to the air, protected by upturned milk crates. Six are in less diverse areas — the edge of a grass oval, a basketball court, a grassy area next to a playground slide, inside school buildings — while five are in the heart of the urban forest.

Every morning and afternoon, a battalion of students – acting as ‘volunteer scientists’ — collect the petri dishes, inscribe the date and location of each collected sample, and put them in the school’s freezer. At the end of each week, the HUMI team picks up the samples and analyses the genetic data back at their labs.
“We have a specific team of kids who are basically the police of this,” Loader smiles. “And they go out every morning and replace them. They feel a great sense of belonging to the team, so they keep other kids away from it.”
When I visit, the experiment has been underway for a month. But James Loader, one of Breed’s students, says they’re already getting data showing a boost in the diversity of soil microbes in Portrush Forest.
That’s one approach. Another is collecting microbes from wilderness areas and then inoculating degraded areas with them. At the Ecophysiology Lab back at the University of Adelaide, research assistant Christian Cando Dumancela shows me soil samples from other sites the HUMI team have been analysing, held in plastic centrifuge test tubes with a conical base.
He holds up three samples: one taken from degraded agricultural land, which is filled with pale, sand-like soil; one from grassland parks where there are no bushes or trees, which is a deeper brown within which soil particles clump together; and the last is from a wilderness site. The latter holds a cornucopia of complexity, including plant and animal residues at various stages of decomposition, rootstalks, a smattering of plant litter, and plenty of humus — the lignins, oils, fats and waxes broken down by microbes.
Cando Dumancela filters the soil to separate out the bacteria from other microbes, then ferments the extracts to grow them, and studies the bacteria with an epifluorescence microscope, which uses filters to separate wavelengths and make the bacteria easier to image. He then sequences the genes of the bacteria, and catalogues the diversity of species found.

“We know that we can bring degraded soils back to health by enriching the soil microbiome, but that can take six to 10 years,” said Breed, holding up the vial with rich wilderness soil. “So, we wondered: can we speed that up? Can we inoculate diverse bacteria in urban soils to regenerate it and bring back biodiversity, and accelerate the complexity of soil microbiome?”
Breed looks at Dumancela, and they both beam excitedly. “No-one has connected highly interventional microbial ecology aimed at improving the health of soils, and the health of people who interact with those soils,” said Breed. “We have. And we think we have a way of speeding that process up.”
But can heathy soils alone make people healthier? The HUMI team have evidence to suggest that ‘microbiome rewilding’ really can achieve this. In a paper published in the international journal Science of The Total Environment in January 2020, they reported on an experiment conducted over seven weeks. In it, 54 juvenile lab mice were kept in wire-top cages, positioned next to an adjoining container of living soil, while small fans occasionally blew a gentle breeze over the soil and into the cages.
One group was next to high-biodiversity wilderness soils, one next to grassland soils, and one next to a clean container with no soil (to mimic an urban setting). Mouse droppings were analysed throughout the process, and their guts sampled at the end of the study. The mice began with similar gut microbiomes, and had exhibited a normal range of anxious behaviour when exposed to standard elevated mazes in their cages.

“The two groups exposed to soil were getting only a dusting — just 0.0034 grams of soil per mouse, per week,” said Breed. “For the ‘wilderness’ mice, their poo showed that their gut microbiome slowly changed to match that of the high-biodiversity soils.
“And we saw two incredible things: a progressive reduction of anxiety behaviours in the ‘wilderness’ mice, as well as a rising abundance of bacterial communities [in their guts] that produce butyrate,” a short-chain fatty acid known in the human and mouse studies to be associated with a balanced immune system, protection from metabolic disorders and reducing rates of anxiety and depression. “And this was from just normal, passive exposure to soil dust in the air.”
By contrast, the no-soil ‘urban’ group showed the most anxiety, while the mice exposed to low-biodiversity soils showed a mix of anxious and non-anxious behaviour in the maze.
“It was unequivocal,” Breed said. “Between 60% and 80% of the variation in anxiety behaviour in the mice could only be explained by the abundance of these butyrate-producing bacteria colonising the gut of the mice. It closed the loop for us between biodiversity exposure and improved mental health. At least in mice.”
WEINSTEIN LEANS forward, his eyes lighting up. “This is the heartland of the research we’re trying to do. We know immune-related disorders like allergies, auto-immune and chronic inflammatory diseases are rising in cities, where the majority of the world’s population now lives. And we know microbial diversity in the gut has immune-boosting power and health benefits. What kind of measures can we take to encourage microbial diversity in environmental microbiomes?

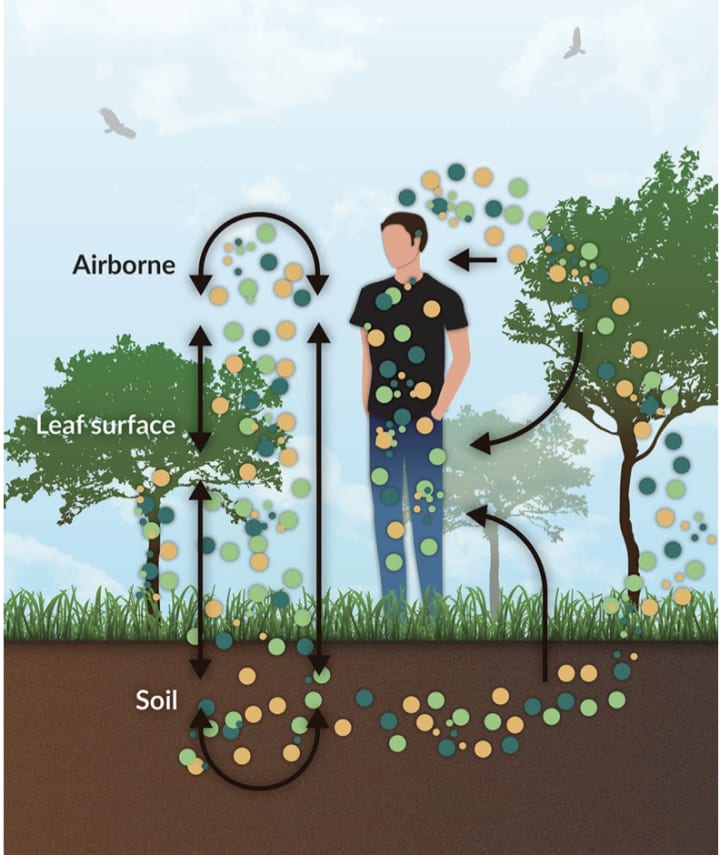
“There’s probably already healthy ecosystem microbes in urban landscapes, but they’re being overwhelmed by these degradation specialists. We just need to help bring those good microbial communities back into dominance,” he said. “We don’t understand all the mechanisms [for why diverse gut microbiomes are heathier], but we know that if you put a biodiverse environment back in an urban space and expose people to it, you will cut out a part of the disease burden.”
“Our first approach is to bring in the kind of [plant] diversity that needs to develop those good microbes. And the second one is to ask — what else can we do to accelerate the process?”
It’s an approach that has captured the interest of the United Nations Environment Program (UNEP) thanks to Weinstein’s colleague, Dr Chris Skelly, a public health specialist in Britain. In November 2018, UNEP signed a partnership with HUMI to apply their approach to 20 cities across 20 countries, in collaboration with the World Health Organisation.
The researchers are also looking at additional approaches — trying to understand which particular microbes provide the most benefits, says Weinstein. “At the other end of the spectrum, we’re asking — which specific bacteria caused this? Can we put them in a pill? If you can marry those two research approaches, we can plant an urban area intelligently to encourage beneficial microbes, and supplement that with key species with a pill if necessary. Then you’ve got the best of both worlds.”
“Adelaide is will be easiest place to start,” says Breed. “We’ve worked with numerous city councils here who are active ibn replanting activities, we’ve got active engagement with the health sector, and there’s also active citizen communities who are interested in doing these programs. A key step for us will be to longitudinally track changes to human health [that result]. Kind of like molecular epidemiology — can we show that the benefits come from the colonisation of people from microbes?”
Andrew Lowe, a professor who coordinates the University of Adelaide’s agricultural research initiatives and has collaborated with the HUMI team, is excited by the possibilities. He said that even though 90% of the natural vegetation around the Adelaide Plains was cleared for farming or urban development, the 10% that remains — like at Mount Lofty — provide vital ecosystem services like bees and other pollinators, water filtration, carbon sequestration, nutrient cycling and nitrogen fixation. These just need to be expanded into urban areas again.


“The good news is that we can return these natural soil microbiomes relatively quickly with a complex set of biodiversity plantings in urban environments,” Lowe said. “You don’t need large areas … once you put the right mix back, then the natural microbiome returns because microbes are highly dispersive, and when they settle in a suitable habitat, they form large colonies and do very well. Even if you do soil inoculations of positive microbes, you still need the plants in those systems for those microbes to survive in the first place.”
It’s an attractive vision: plant diverse species of trees, shrubs, herbs and grasses in open spaces –such as parks, along train lines, electricity corridors and the edges of highways — that not only make cities greener on the outside, but make us healthier on the inside. And maybe bring a little piece of Mount Lofty into every suburb.
Like this story? Please click the ♥︎ below, or send me a tip. And thanks 😊
About the Creator
Wilson da Silva
Wilson da Silva is a science journalist in Sydney | www.wilsondasilva.com | https://bit.ly/3kIF1SO






Comments
There are no comments for this story
Be the first to respond and start the conversation.