Sublimation
Introduction, principle, procedure and its application.
SUBLIMATION
Introduction
A substance can shift from a solid to a gaseous state without first transitioning to a liquid form through a process known as sublimation. Sublimation completely avoids the liquid phase, in contrast to melting or vaporization, which require transitions between the solid and liquid, and the liquid and gas stages, respectively.
The solid substance absorbs enough energy during sublimation to dissipate the intermolecular forces holding its particles together. The particles then acquire enough kinetic energy to escape the solid lattice and transition into the gas phase. This process takes place at pressures and temperatures when the substance's vapour pressure is greater than the air pressure in the area.
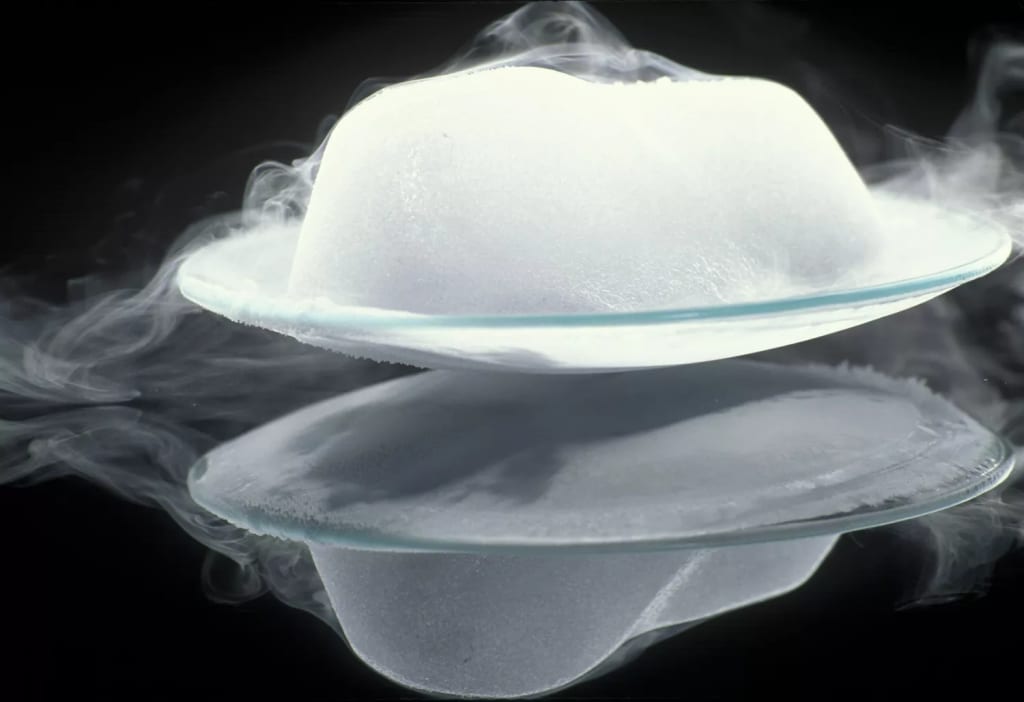
Many substances, including dry ice (solid carbon dioxide), mothballs (naphthalene), and iodine crystals, exhibit sublimation often. When subjected to the proper circumstances, these substances can convert instantly from a solid into a gas. Deposition refers to the opposite process, in which a gas transforms immediately into a solid.
In addition to freeze-drying, sublimation has use in chemistry, material science, and even commonplace activities. Among other things, it is used to manufacture semiconductor devices, make thin films, purify certain compounds, and make air fresheners.

PRINCIPLE
It is founded on the idea that solids can go from a solid state directly into a gaseous one without first going through a liquid stage.
The thermodynamic idea that a substance can move straight from the solid phase to the gas phase without going via the liquid phase forms the basis of the sublimation principle. The interaction of temperature, pressure, and the substance's vapour pressure causes this process to take place.
The phase diagram of a substance, which depicts the correlation between temperature and pressure for several phases (solid, liquid, and gas), can be used to demonstrate the principle of sublimation. The sublimation curve is one of the transition points between various phases that is commonly depicted in the phase diagram.
The substance is solid at pressures and temperatures below the sublimation curve. The particles are held together tightly in this condition by the intermolecular interactions, creating a stable lattice structure. The temperature of the solid material rises as heat is supplied, and the particles acquire kinetic energy and begin to vibrate more quickly within the lattice.
Sublimation takes place when the substance's vapour pressure exceeds the ambient atmospheric pressure due to changes in temperature and pressure. The particles can escape the solid lattice and reach the gas phase directly thanks to their enhanced kinetic energy, which allows them to overcome the intermolecular interactions. This shift is caused by the substance's propensity to reach a more energetically advantageous state.
The sublimation principle can be summed up as follows:
1. When first introduced, the substance is solid.
2. The solid substance is heated, raising its temperature.
3. The substance's vapour pressure exceeds atmospheric pressure at a particular temperature and pressure combination.
4. The solid particles acquire sufficient energy to escape the solid lattice and enter the gas phase right away.
5. The gas specks scatter throughout the atmosphere.
It's significant to remember that not all chemicals sublimate under typical circumstances. The likelihood of sublimation is influenced by a number of variables, including the molecular make-up of the substance, intermolecular forces, and the temperature and pressure levels.
PROCEDURE
Chemists utilize the sublimation process to clean up their chemicals. Usually, a solid is heated under vacuum in a sublimation equipment. A non-volatile residue of impurities is left behind as the solid volatilizes and condenses as a refined chemical on a cooled surface (cold finger) at this lower pressure.

APPLICATION
There are several uses for sublimation in different industries. In the process of freeze-drying, for instance, moisture is removed from food, medicines, and other perishable commodities while maintaining their quality. This procedure involves freezing the material, followed by applying less pressure, which causes the ice to melt and leave behind a dehydrated product.
The process of freeze-drying, which is used in the frozen food sector, is a significant use of sublimation. When we lower the pressure around the substance, the frozen water sublimates from the solid phase to the gas phase.
Ablation, a process that wears away glaciers, is brought on by erosion and sublimation.
Iodine sublimation can be used to make latent fingerprints on paper visible.
• Compounds can be purified by sublimation.
• Since dry ice sublimates so easily, the substance is utilised to simulate fog.
• In general, sublimation is a remarkable process that enables some materials to pass immediately from the solid to the gas phase, providing uncommon chances for scientific investigation and useful applications.




Comments
There are no comments for this story
Be the first to respond and start the conversation.