Crystallization
Introduction, types and its applications.

Crystallization
Introduction
The process of creating crystals from solutions is known as crystallization.
Introduction: The production of crystals—well-defined, organized arrangements of atoms, ions, or molecules—is a basic process in chemistry and material science. When a solute precipitates out of a solution, solid crystalline structures start to develop as a result.

The crystallization process normally entails the following crucial steps:
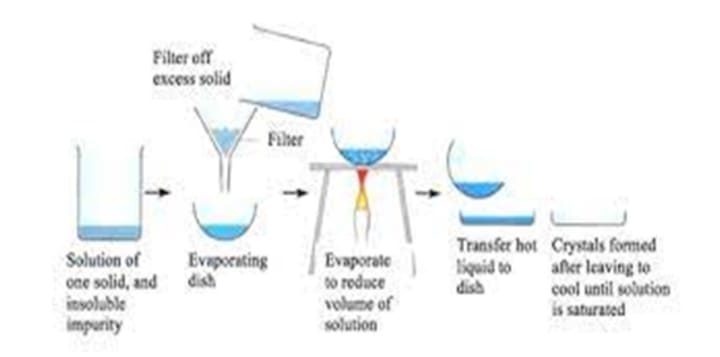
The process of creating a solution involves dissolving the solute (solid) in an appropriate solvent (liquid). The solute should only be partially soluble in the selected solvent at the desired temperature.
Nucleation: In a supersaturated solution, nucleation is the first creation of microscopic clusters of solute particles known as nuclei. Primary (homogeneous) nucleation, which happens naturally, and secondary (heterogeneous), which is aided by outside particles or surfaces, are two different types of nucleation.
Crystal Growth: As more solute particles adhere to the surfaces of produced nuclei, they enlarge in size. The solute molecules or ions diffuse from the solution to the crystal lattice during this growth phase.
Crystalline Solid Formation: The solute particles reorganize into an organized, repeating pattern as crystal growth progresses, generating a solid crystal structure.
Elements That Affect Crystallization
The crystallization process and the characteristics of the resulting crystals are influenced by a number of factors:
Supersaturation is a critical component of crystallization and is attained by dissolving more solute than the solvent can typically hold. Adjusting variables like temperature, pressure, and solute concentration can influence it.
Cooling Rate: The rate of crystal development is influenced by the rate at which the solution is cooled. While quick cooling frequently produces smaller crystals, slow chilling permits the formation of bigger crystals.
Stirring and Agitation: During the crystallization process, stirring or agitating the solution promotes nucleation and inhibits the growth of big, unfavourable crystals.
Impurities: Impurities in the solution might impair crystal development and the purity and quality of the crystals that are produced as a result. Impurities may interfere with crystal development or incorporate into the crystal lattice.

Crystallization types: Depending on the conditions and methods utilized, there are many forms of crystallization:
Evaporative crystallization
This process includes slowly removing the solvent from the solution, which causes supersaturation and crystallization to occur. It is frequently employed to obtain some chemical compounds and salt crystals.
Cooling Crystallization:
As solubility declines with decreasing temperature, supersaturation comes from cooling a hot, saturated solution. In both industrial and laboratory procedures, this method is frequently employed to produce crystals.
Solvent Crystallization:
To cause supersaturation and crystal formation, solvent crystallization entails altering the solvent's properties, such as solubility or polarity. It is used in methods for separation and purification.
Slow Crystallization:
This process promotes the development of big, distinct crystals by allowing the solution to cool or evaporate slowly over an extended period of time. In investigations on crystal formation and crystallography, this method is frequently used.
Applications
Crystallization has a wide range of applications in several fields, including:
Chemical Synthesis: Crystallization is frequently used to isolate and purify chemical molecules that have been created in the lab. It enables the creation of superior, pure compounds with distinct crystal formations. This is crucial for the pharmaceutical and fine chemical industries since a compound's efficacy and purity can be greatly impacted by its purity and crystalline shape.
Pharmaceutical Industry: The manufacture of pharmaceuticals depends heavily on crystallisation. Active pharmaceutical ingredients (APIs) are purified and turned into crystalline solids using it. The stability, solubility, bioavailability, and other qualities of a drug's crystalline form can have an impact on its efficacy and manufacture.
In the field of material science, crystallization is crucial for the creation of sophisticated materials with particular features. Researchers can create materials with certain features, such as improved strength, optical capabilities, electrical conductivity, or magnetism, by manipulating the crystallisation process. Examples include the creation of single crystals for X-ray diffraction analysis and semiconductor crystals for electronics.
The food and beverage industry:
Crystallization is frequently used in this sector. It is employed in the manufacturing of chocolate, confectionery goods, and sugar crystals. The manufacturing of salt, flavourings, and food additives all depend on carefully controlled crystallization to provide the proper texture, look, and quality of the finished goods.
Energy Storage: Applications for crystallization include energy storage, particularly in the area of rechargeable batteries. During charge and discharge cycles, the electrode materials in batteries frequently go through processes of crystallisation and dissolution. It's imperative to comprehend and manage these processes if you want to increase the performance, capacity, and lifespan of your batteries.
h. Nanotechnology: In order to create nanoparticles and nanomaterials, crystallization processes are also used in this field. Researchers may create nanoparticles with specified sizes, shapes, and crystalline structures by manipulating the crystallisation conditions. This enables the creation of sophisticated materials with distinctive capabilities for use in a variety of fields, including catalysis, electronics, and biomedicine.
In conclusion, crystallization is a flexible process with several applications in a variety of industries. It makes it possible to create pure chemicals, create cutting-edge materials, purify mixtures, and investigate geological and chemical processes. Its applications are anticipated to grow further with continuous research and improvements in crystallization procedures, supporting scientific and technological developments.





Comments
There are no comments for this story
Be the first to respond and start the conversation.