Spectroscopy
Introduction, principle, working, types and application
1 SPECTROSCOPY
In physical and analytical chemistry, spectroscopy is used to identify, ascertain, or quantify the molecular and/or structural makeup of a material. Atoms and molecules of various types will each reflect, absorb, or emit electromagnetic radiation in ways that are unique to them.
1.1 PRINCIPLE
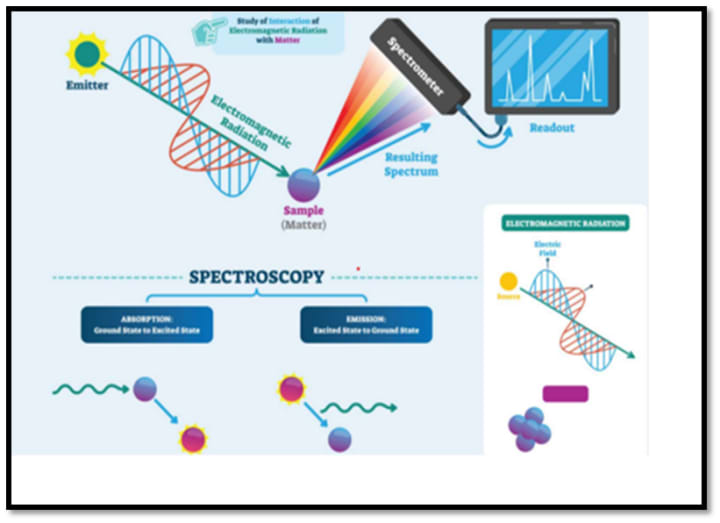
The fundamental idea behind all spectroscopic techniques is to expose a sample to an electromagnetic field and then see how it reacts. Typically, the response is measured in relation to the radiation wavelength.
1.2 SPECTROPHOTOMETER
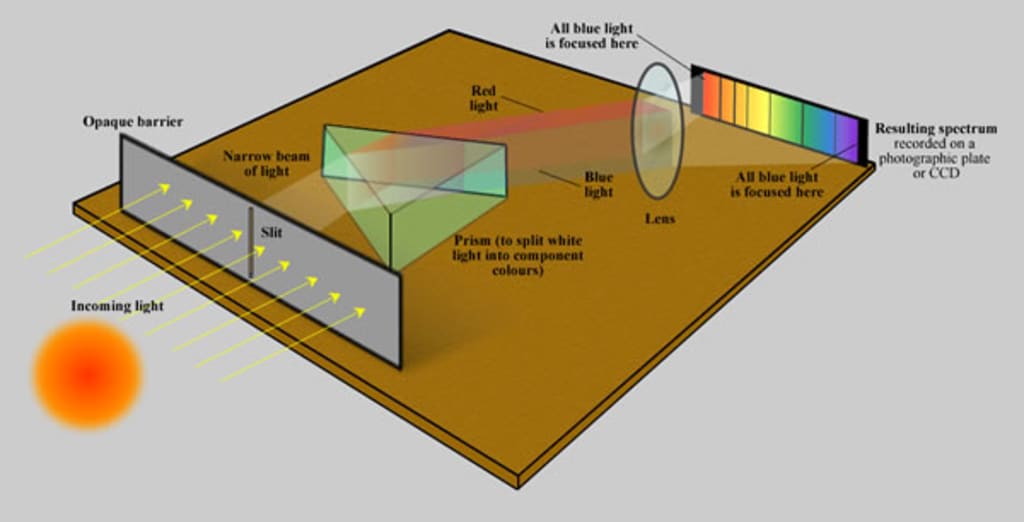
The intensity of electromagnetic energy at each light wavelength in a given location is measured by a device called a spectrophotometer. A UV-visible-NIR spectrophotometer operates in the ultraviolet, visible, and near infrared spectrums, like those found in CRAIC microspectrophotometers.
1.3 WORKING
A light source, a way to direct light onto the sample, a technique to gather light from the sample, a monochromator to break the light down into its individual wavelengths, and a detector to gauge the strength of light at each wavelength make up a spectrophotometer.
1.4 TYPES
On the basis of their underlying principles, spectroscopic techniques come in a wide variety. The different categories of spectroscopy are as follows;
• Spectroscopy in the infrared (IR)
• UV/Vis (ultraviolet-visible) Spectroscopy
• Spectroscopy using nuclear magnetic resonance (NMR)
Raman and X-ray spectroscopy are two examples.
Depending on the method of sample preparation, etc., each primary type of spectroscopy has numerous more subtypes.
1.5 INFRARED SPECTROSCOPY
The electromagnetic spectrum of infrared light is measured via infrared spectroscopy. The most notable feature of IR spectroscopy is how effective it is at determining the functional groups of organic compounds. Molecular vibrations are produced when infrared light is absorbed by organic molecules. Each functional group has its own set of vibrational frequencies. Graphs of IR spectra are presented as transmittance (%) vs. wavenumber (cm-1).
1.6 X-RAY CRYSTALLOGRAPHY
A crystalline molecule's structure can be examined via X-ray crystallography. Each atom's electron cloud diffracts X-rays, which reveals the positions of the atoms. DNA, proteins, salts, metals, and other organic and inorganic compounds can all crystallise and be utilised in this process. The sample that is utilised for analysis is kept.
1.7 UV SPECTROSCOPY
In order to determine how much of a chemical is present in a liquid, ultraviolet-visible spectroscopy uses both visible and ultraviolet light. The UV-Vis method is based on the colour of the solution. The chemical makeup of the solution we are working with gives it a certain colour. The light it reflects is the colour of the solution since the solution absorbs some light colours while reflecting others. By shining light through a sample of your solution, UV-Vis spectroscopy measures how much light is absorbed by the solution.
1.8 NUCLEAR MAGNETIC RESONANCE
Nuclei can be observed via nuclear magnetic resonance. It makes use of some nuclei's magnetic properties, primarily those of 13C and 1H. The NMR device produces a strong magnetic field that causes the nuclei to behave like little bar magnets. Either the nuclei align with the magnetic field of the device or they do not. We currently have two options for the nuclei's orientation: or. The nuclei are then exposed to radio waves, which cause them to rotate. An energy release and detection occur when this transition takes place. A computer system visualises the data (Intensity vs. chemical changes in ppm). Your analytical sample won't be ruined by NMR. A 900 MHz NMR apparatus is shown below.
1.9 APPLICATIONS
The following are some uses for spectroscopy:
figuring out a sample's atomic structure
figuring out a muscle's metabolic structure
monitoring the concentration of dissolved oxygen in freshwater and marine ecosystems
studying the spectral emission lines of distant galaxies
Characterizing proteins
Space exploration; and respiratory gas analysis in hospitals.
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.




Comments
There are no comments for this story
Be the first to respond and start the conversation.