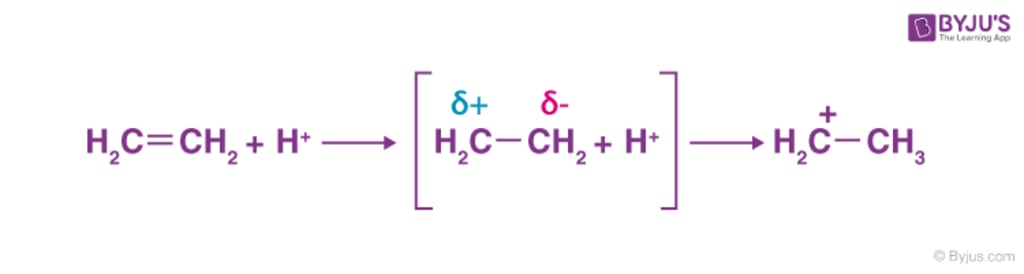
Electronic effect (also known as the "emission effect")
Definition
The transfer of electrons from low electronegative atoms to high electronegative atoms is how it is described.
OR
The term "electronic effect" refers to a change in the distribution of electrons (bonded or unbonded; bonded electrons may be sigma or pi-electrons) in a covalent bond of any chemical that affects that compound's behaviour, characteristics, stability, and reactivity.
Permanent and temporary electronic effects are two different types of effects.
Electronic effect that lasts forever
That kind of effect is brought about by the constant shifting of electrons that takes place in the ground state when an atom or substituent group is present.
Example:
Sigma effect refers to an atom or group's ability to permanently transfer sigma electrons away from the carbon atom.
For instance, NO, CN, COOH, F>-CI, and Br>-I>-OH.
+ effect: Sigma electrons are permanently shifted in the direction of the carbon atom as a result of an atom or group.
1 Inductive result
The inductive effect is the movement of sigma-electrons along a saturated carbon chain as a result of a polar covalent bond existing at one end of the chain.
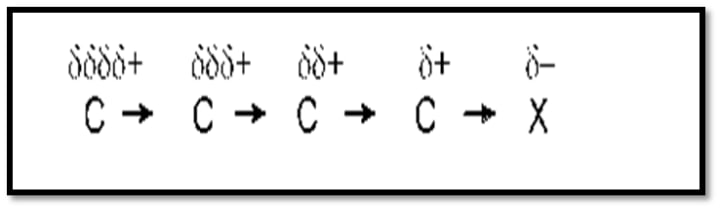
Example:
The C-Cl bond in butyl chloride is bipolarized due to a variation in electronegativity. Carbon atoms lose their electrons when exposed to chlorine. The initial carbon atom thus receives a partial positive charge.
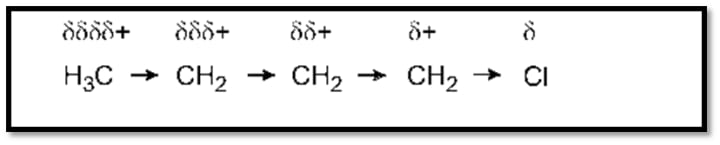
The inductive effect is characterized by the following characteristics: It exclusively affects sigma bonds, lasts for a maximum of the second carbon atom in the chain, and ceases to function after the fourth.
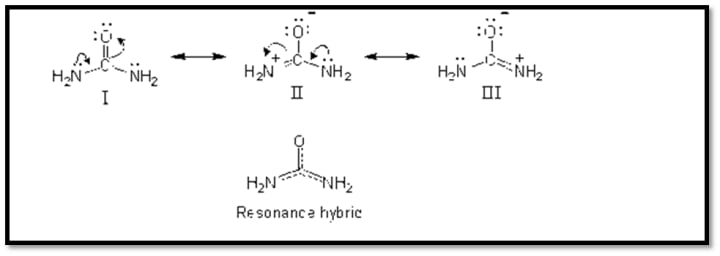
2-Resonance or Mesomeric phenomenon:
A form of electronic phenomenon brought on by the complete delocalization of pi-electrons or lone pair electrons that move away from or towards the substituent in the conjugated orbital arrangement.
Examples:
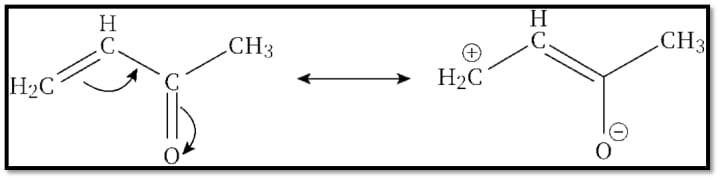
Resonance structure is found in urea:
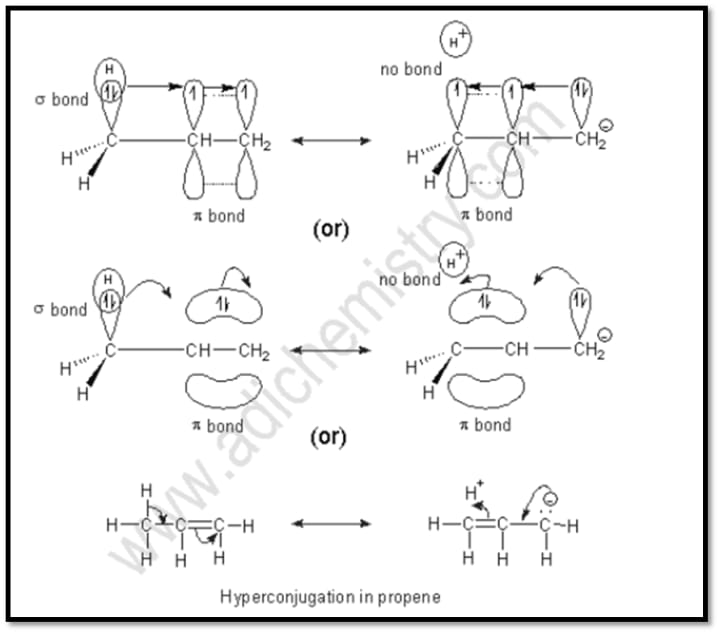
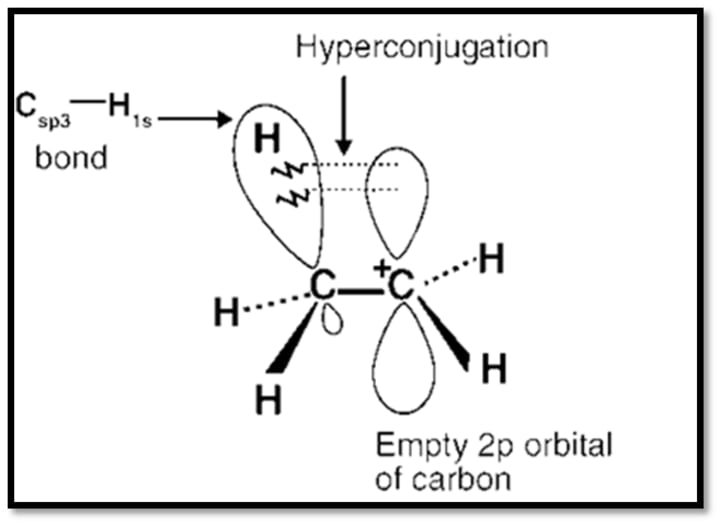
3- Hyperconjugation:
The electrical result of the C-H bond's partial delocalization (sigma-electrons).
Instantaneous electronic effect
The kind of electrical effect that reagents have on electronics.
Unsaturated molecules, such as those with numerous bonds (double or triple covalent compounds), can have this effect.
1 — Electromeric impact
The occurrence of a shared pair of pi-electrons completely transferring from one atom in a multiple bond to the more electronegative atom of the bound atoms when an attacking reagent is required is known as the electromeric effect.
2-Inductomeric effect:
It is a type of temporary effect that enhances the inductive effect and it is found only in the presence of an attacking agent.
Example:

Due to the - I effect of Cl group in methyl chloride further increases the effect temporarily by the reach of hydroxyl ion.
Prediction of acidity using inductive effect:
Groups that have -I effect on a molecule decrease its electron density that make the molecule electron deficient and more acidic.
It means that the acidity of a compound is directly proportional to the number of electron withdrawing groups.
Example:
CH3COOH < CH2FCOOH < CHF2COOH < CF3COOH.

Strength of benzoic acids and aliphatic carboxylic acid:
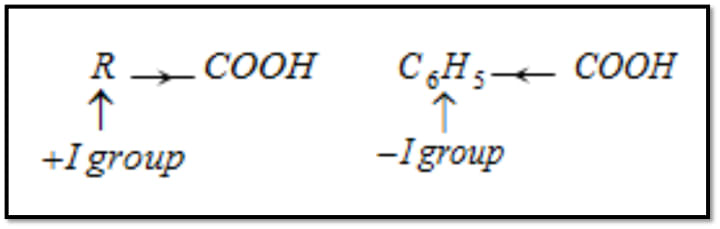
Hence benzoic acid is stronger acid than aliphatic carboxylic acids but exception is formic acid.
Acidic strength order of mono, di and triacetic acid is as follows:
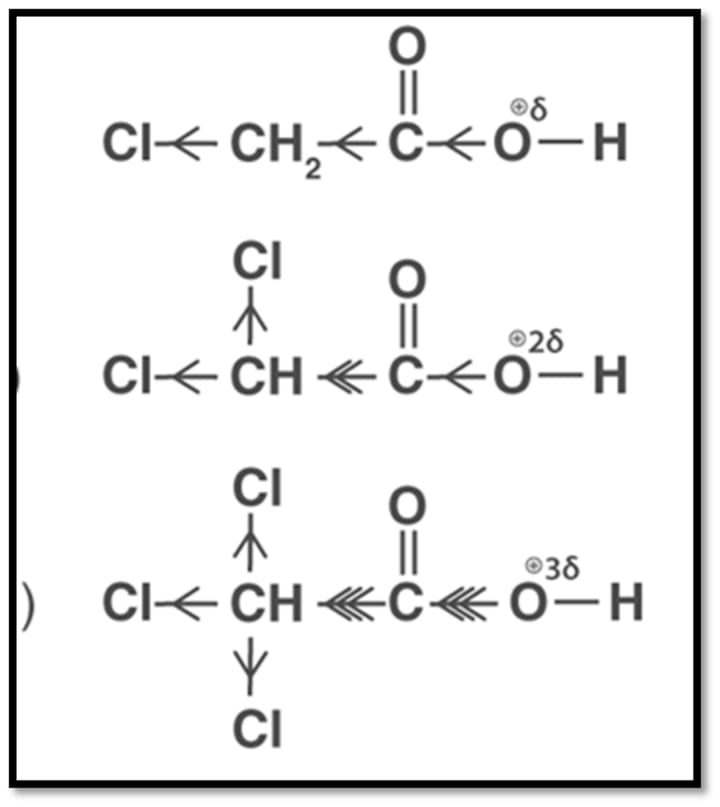
Prediction of acidity using Resonance:
Stable conjugate base of a resonating structures will enhance the acidic strength.
Example:
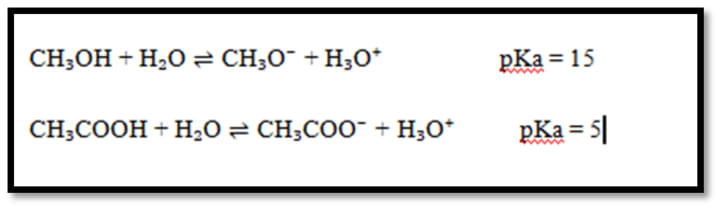
Explanation:
Conjugate base of acetic acid is more stable than methanol so it is 1010 times more acidic than methanol. Negative charge on the oxygen atom is localized in methoxide ion.
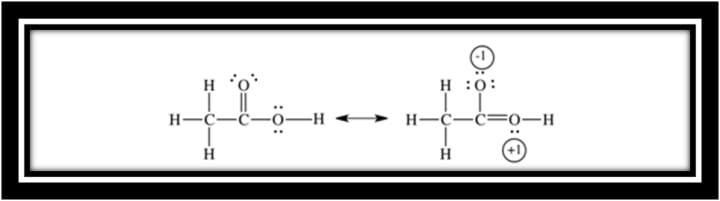

On the three atoms the negative charge is delocalized. That delocalization creates a lower-energy state.
Prediction of acidity by hyperconjugation:
Acidity of an organic compound is increased by increasing the stability of conjugate base of an acid. So, hyperconjugation plays an important role in increasing that stability of conjugate base.
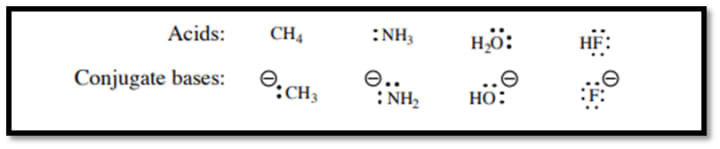
The actual pKa values are of CH4 is 51 (weakest acid), NH3 pKa 38, H2O pKa 15.7 and HF pKa 3.2.
Prediction of basicity using inductive effect:
Groups that have +I effect attached to a molecule increases the over all electron density on the molecule and the molecule is able to donate electrons, making it basic.
It means that the electron-donating group increase the basicity of a compound.
Example:
Due to the +I effect of methy group in acetic acid, acetic acid is more basic than formic acid and formic acid is more acidic than acetic acid.
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.




Comments
There are no comments for this story
Be the first to respond and start the conversation.