A Not-So-Selfish 'Genetic Parasite' Preserves Fertility
Unraveling the Mystery: How Retrotransposons Preserve Fertility and Maintain rDNA

Ribosomal DNA (rDNA) sequences play a vital role in the genetic makeup of numerous species. However, the repetitive nature of these DNA sequences makes them prone to shrinkage over time, endangering cell survival. When rDNA shrinks significantly in germ cells, the cells responsible for producing eggs and sperm, individuals may face infertility, and entire lineages could face extinction. Scientists have long sought to unravel the mystery behind the preservation of rDNA across generations. Recent research conducted by Whitehead Institute Member Yukiko Yamashita and postdoc Jonathan Nelson has uncovered an unexpected guardian of rDNA—retrotransposons, genetic elements previously labeled as genetic parasites due to their tendency to replicate themselves.
The study, published in the journal PNAS on May 30, 2023, sheds light on how these so-called parasites fulfill an essential role in maintaining rDNA and safeguarding fertility over generations.
The Puzzle of rDNA Persistence

rDNA serves as the blueprint for generating RNA subunits in ribosomes, the cellular machinery responsible for protein synthesis. As cells require a significant number of ribosomes to produce the necessary proteins for proper functioning, rDNA contains numerous repeated copies of the ribosome production sequence.
The challenge lies in the repetitive nature of this DNA. During cell division and genome replication, the identical repeats are vulnerable to accidental removal. Over time, as cells undergo multiple divisions, the number of repeats naturally diminishes.
This phenomenon is particularly noticeable in aging cells and germ cells—the only cells passed from one generation to the next. If no restorative mechanism for rDNA repeats exists, each subsequent generation would possess fewer repeats than the previous one, eventually leading to insufficient repeats for viable germ cell production and population decline.
Yamashita, a professor of biology at the Massachusetts Institute of Technology, focuses on studying germ cell immortality in male fruit flies. Her research delves into how germ cells can continuously generate healthy sperm and eggs across multiple generations.
While other cell types perish with the body, accumulating some genetic damage over time, germ cells must diligently maintain their rDNA to preserve immortality. Despite the acknowledgment that something sustains rDNA, the specific protective mechanism remained unknown until now. Nelson and Yamashita embarked on a mission to unravel the mystery.
Retrotransposons: More than Selfish Parasites
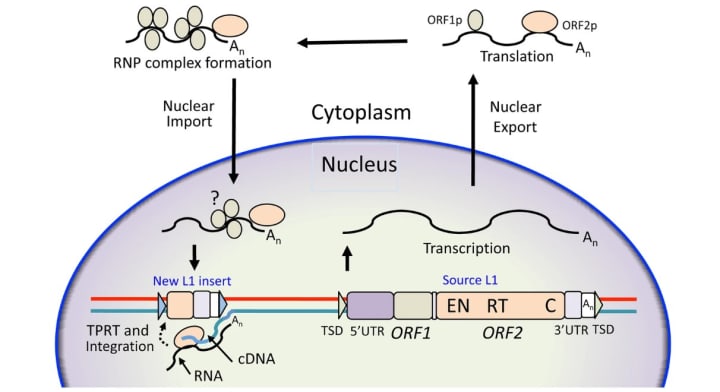
Their groundbreaking research reveals that rDNA is rejuvenated with the assistance of a retrotransposon known as R2. Retrotransposons are genetic sequences primarily driven to self-replicate, even at the expense of the host genome. Due to their replication-focused behavior, they were previously considered genetic parasites. However, their actions closely resemble those of viruses, which manipulate cells into producing copies of themselves. Retrotransposons achieve this by reversing the conventional process of gene expression.
When the DNA encoding a retrotransposon is transcribed into RNA, the RNA can then be converted back into DNA. The retrotransposon proceeds to cleave open the cell's genome and insert its new DNA, thereby adding another copy of itself to the genome. This process not only expands a species' genome size over generations—nearly half of the human genome consists of transposable elements—but also poses a risk of cellular damage.
When a retrotransposon disrupts the genome, particularly if it inserts itself into a critical DNA sequence, essential genes can become inoperable.
However, Nelson and Yamashita discovered that the retrotransposon R2, which typically copies and inserts itself into fruit fly rDNA, can also provide benefits to cells. During cell division, each chromosome is duplicated to distribute one copy to each new daughter cell. R2 cleaves both copies of the chromosome containing rDNA. When the cell attempts to repair these breaks, the repetitive nature of rDNA causes it to lose its original structure. Consequently, the cell stitches a stretch of rDNA repeats from one copy of the chromosome into the other copy.
This process results in one daughter cell possessing more rDNA repeats than the original cell, while the other daughter cell has fewer repeats. Germ cells ensure their immortality by utilizing the daughter cell with a greater number of rDNA repeats to perpetuate the germline.
Another study conducted by Yamashita's lab in 2022 identified a gene named Indra, responsible for producing a protein that binds to the chromosome containing more rDNA repeats. This protein marks the daughter cell with additional repeats as a stem cell, ensuring its continuation, while the other daughter cell proceeds toward sperm production.
Germ cells employ a combination of mechanisms, transferring rDNA repeats from one chromosome to another, and selectively marking cells with greater repeats. This constant replenishment of rDNA levels in the germline ensures that the population of germ cells maintains a sufficient number of rDNA repeats, securing the lineage of the cells and the individuals carrying them.
Nelson and Yamashita's research underscores that R2 retrotransposons are not mere selfish parasites; they play a pivotal role in the rejuvenation of germline rDNA. However, being retrotransposons, R2 elements also possess the capacity to induce damage. Nelson discovered that germ cells keep R2 inactive unless the number of rDNA repeats drops to a critically low level.
In this way, cells can maximize the benefits of R2 while minimizing potential risks, activating retrotransposons only when necessary. This strategic approach allows cells and retrotransposons to establish a mutually beneficial relationship. Yamashita and Nelson speculate that other transposable elements may also provide unknown advantages to the cell.
Many transposable elements are commonly viewed as existing due to their superior replication ability compared to the host's defense mechanisms. These elements occupy significant regions of the genome that are often perceived as non-functional. However, what if the abundance of these elements stems from their contribution to cellular functions that are currently not fully understood?
In conclusion, the collaborative research conducted by Yukiko Yamashita and Jonathan Nelson sheds light on the vital role played by retrotransposons, particularly the R2 element, in the preservation of rDNA and fertility across generations. These findings challenge the conventional notion of retrotransposons as selfish parasites and highlight the potential benefits they offer to the host cell. The intricate mechanisms employed by germ cells to maintain rDNA levels ensure the perpetuation of viable germ cells and the preservation of lineages. Further exploration of transposable elements may reveal additional functions that have eluded scientific understanding thus far.
References:
(Phys org [https://phys.org/news/2023-06-not-so-selfish-genetic-parasite-fertility.html)
About the Creator
satish Kumar
Content Strategist, YouTuber, Website Developer & SEO Analyst: Dedicated to Constant Skill Growth






Comments
There are no comments for this story
Be the first to respond and start the conversation.