Who May Apply for Approval of Non-Specified Foods?
Important Factors Of Non Specified Food Approval
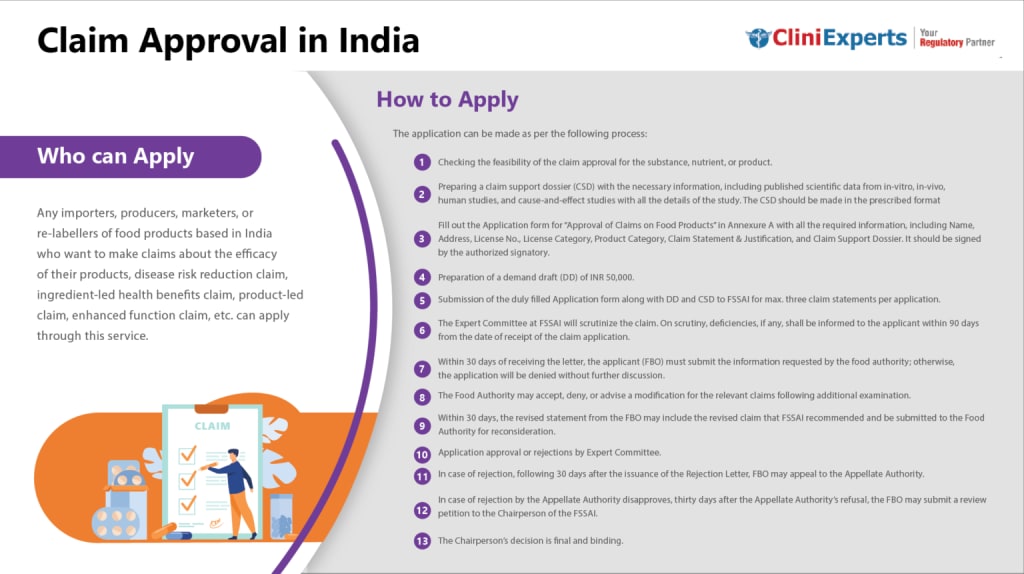
Non-Specified Food Approval may be required by various organizations involved in the development, production, and distribution of functional foods in the functional food industry.
The qualification models rely upon the particular job inside the practical food business. A breakdown of who can apply is as follows:
Understanding Non-Specified Foods
Non-specified foods are those that do not fit into the existing categories defined by the FSSAI. These can include innovative food products, new food additives, processing aids, or foods that use new technologies. Because these items do not have specific regulatory guidelines, they require special approval before being marketed.
Eligible Applicants
The following entities are eligible to apply for the approval of non-specified foods:
Food Business Operators (FBOs):
Manufacturers: Companies that produce non-specified food items and wish to introduce them to the market.
Importers: Businesses that want to import non-specified food products into India for sale.
Food Researchers and Innovators:
Research Institutions and Universities: Organizations involved in food research and development may apply for approval if they develop new food products.
Individual Innovators: Individuals who have developed a unique food product can also apply for approval.
Food Additive and Processing Aid Developers:
Companies or individuals who create new food additives, processing aids, or technological advancements in food processing can seek approval for their products.
Application Process for Non-Specified Foods
Preparation of the Application:
The applicant must prepare a detailed application that includes comprehensive information about the non-specified food item. This should cover the composition, intended use, safety data, and any available literature or research supporting the safety and efficacy of the product.
Submission to FSSAI:
The application must be submitted to the FSSAI along with the requisite fees. The application can be submitted online through the FSSAI’s Food Import Clearance System (FICS) or directly to the concerned department handling non-specified foods.
Review and Assessment:
Upon receiving the application, FSSAI will conduct a thorough review. This includes assessing the safety data, evaluating the potential health impacts, and ensuring the product complies with general food safety standards.
Expert Panel Evaluation:
In some cases, FSSAI may refer the application to an expert panel for detailed scrutiny. The panel may include food safety experts, toxicologists, nutritionists, and other relevant professionals.
Approval or Rejection:
Based on the review and recommendations of the expert panel, FSSAI will either approve or reject the application. If approved, the applicant will receive a certificate of approval, allowing them to market the product in India. If rejected, the applicant will be provided with reasons for the rejection and may be given an opportunity to address any concerns and reapply.
Utilitarian Food Makers
- Elements Included: Organizations or associations participated in the creation of practical food sources that don't fall under existing food classifications.
- Necessities: Need to apply for endorsement to guarantee the security and viability of new practical fixings or new purposes of existing fixings.
Entities Involved in the Development of Novel Foods:
- Companies creating novel food products using novel technologies or ingredients.
- Necessities: Should go through a particular assessment to show the security and advantages of the new items before they can be promoted.
Food Shippers and Exporters
- Elements Included: Organizations engaged with the worldwide exchange of utilitarian food varieties.
- Prerequisites: Need to acquire Non-Determined Food Endorsement for bringing in or sending out useful food sources that are new to the market or ward.
Retailers and Merchants
- Substances Included: Corporate store, wellbeing food stores, and wholesalers managing new useful food items.
- Prerequisites: must ensure that all functional foods sold have been approved by the appropriate regulatory agency.
Research Organizations and Colleges
- Elements Included: New functional food ingredients or products are being developed by educational and research institutions.
- Necessities: should submit an application for approval before beginning research on novel functional foods in order to ensure compliance with safety regulations.
Sponsors of Clinical Trials
- Involved Organizations: organizations that are testing new functional foods in clinical trials to back up health claims.
- Necessities: must get the licenses and approvals they need to legally run clinical trials and provide data to back up functional claims.
Showcasing Approval Holders
- Substances Included: Organizations liable for showcasing and circulating practical food varieties inside a particular locale.
- Prerequisites: need to get Non-Specified Food Approval to make sure that the products that are sold meet the safety and effectiveness standards set by the government.
Food Repackagers
- Participants: Organizations repackaging practical food sources for circulation under their own name.
- Prerequisites: Should apply for endorsement to guarantee the repackaged items conform to administrative norms and keep up with their wellbeing and viability.
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.





Comments
There are no comments for this story
Be the first to respond and start the conversation.