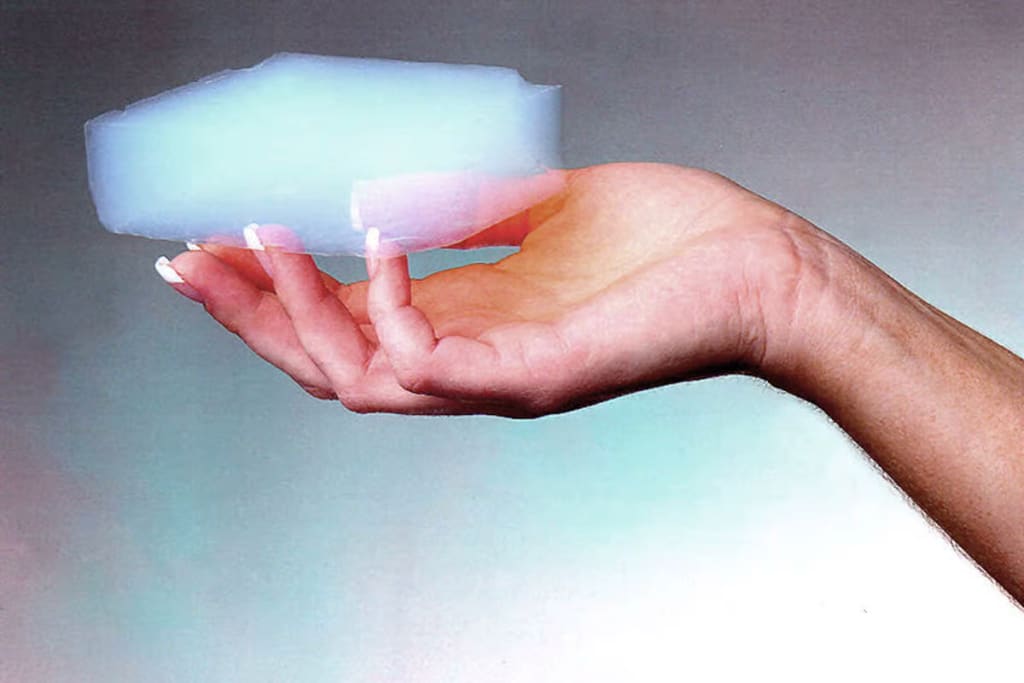
It's aerogel. the least dense and lightest solid known to man.
This object weighs only 1.22 grammes.
Given that it is 99.8% air, the mass of that object is only a fraction of the mass of the same volume of air. In fact, certain aerogels are so light that they would be less dense than air if all the air were taken out of them. I really flew to Aerogel Technologies in Boston to learn more about the history of aerogel since I have been captivated by it for a very long time.
How is it produced?
What is it used for, and why is it such a superb thermal insulator?
So, over here, we have two setups: one with a glass petri dish and the other with aerogel on top. We're going to perform an experiment to show the effectiveness of aerogel as an insulator.
Despite having quite different physical structures, both are comprised of silica.
We're going to use a Bunsen burner to melt these chocolate bunnies and observe how long it takes.
We have a FLIR T1020 that can detect temperatures up to 2,000 degrees Celsius to take a closer look at this experiment.
It appears to be getting quite hot.
Yes, you can see that the glass is already really warm. And barely a minute later, it begins to smoke. Okay. Without a doubt, it is smoking and melting.
Oh, okay, let's begin.
That, in my opinion, is a phase transition.
A situation involving liquid chocolate exists.
There is some smoking rabbit here. The bunny is actually melting over here, and see, it's sort of tipping to the side over there.
Okay, I believe we should refer to that as a melt.
Whoa, what's that? - Oh!
on time, on time.
That is a major failure, in my opinion.
The petri dish split as a result of the temperature expansion, in addition to the rabbit melting quickly.
Want to plug it in?
Let's try the aerogel now.
How was aerogel created in the past?
Professor Samuel Kistler and his colleague Charles Learned engaged in a wager in 1931.The wager is now focused on jellies, specifically peanut butter and jelly jellies.
Jellies are unique in that they actually consist of both liquids and solids.
They're essentially liquid, after all, but they're enclosed in this three-dimensional solid structure.
Therefore, if you imagine a gel like jelly having a skeleton made of nanoscale pores that provides it stiffness, that would make up around 1% of the gel.
So the question was, "Could you get the liquid out of the jelly?" without changing the sturdy construction? If you simply evaporate the liquid, then
As liquid molecules are removed, they pull on one another and the surrounding solid structure, essentially crumpling it from the inside and causing the solid structure to shrink.
Samuel Kistler has found two solutions to resolve this issue.
First, he understood that you could just thoroughly wash the jelly to replace one liquid with another. So, for example, you could substitute alcohol for water.
And if you take the jelly and place it in an autoclave, a high-pressure vessel.
The liquid was made into a semi-liquid, semi-gas known as a supercritical fluid by heating it to the high-temperature, high-pressure point known as the critical point of the liquid.
There is no longer a difference between liquid and gas at this point.
The forces between the molecules have stopped.
As a result, after depressurizing the vessel, only 1% of the gel's mass and the solid skeleton remain intact.
This solid skeleton, a nanoporous solid that we refer to as aerogel, is what was formerly liquid but is now gas.
In Nature, Kistler published his findings in 1931.The thermal camera shows that it is getting quite warm.
But three minutes have almost passed with no indication of melted chocolate.
We will thus take a thermocouple out and just check the temperature there.
See what the flame temperature is like underneath the aerogel.
It's kind of obvious that the bunny is heating up in some places, but it's not just at the bottom; it's all around the bunny.
Exactly. You can see the aerogel is getting very hot because the convective heat is going up and around it.
By four minutes, the bunny was beginning to appear a little frail.
But considering how simple it is to melt chocolate, it's still really delicious.
Possibly putting my finger here be cautious.
It's brittle, not heated, which is why it's a problem Right.
However, it's still really cool to touch, right? - It is, but only to a warm degree.
He created aerogels using a variety of materials.
He created them from eggs. He created them from nitrocellulose and rubber.
And silica was contained therein. I actually have some instances of silica gel sitting here on the table.
Wet silica gel is what this is.
Since it has a rubbery texture, I can simply slice out a piece.
Within its pores, there is 97% alcohol.
Amorphous silica makes up the remaining 3% of the solid. Just a moment, may I touch it? - Yes, without a doubt.
It has a rubbery texture.
Not very powerful.
Was it already cracked there, or was I just starting to crack it there?
Yes, you just, no-- Oh my goodness, it's so simple to break. Very brittle.
The gel's alcohol needs to be replaced with liquid carbon dioxide in the next step.
We're going to see fluid CO2.Fluid CO2 enjoys the benefit of being non-combustible, in addition to having a low basic temperature.
If you open it, you'll see that it's flooding in.
Definitely, it's flooding in. It moves there. A single more solvent.
You can plainly see that it's such a great deal cooler on top.
What temperature does the bottom have? We're currently at 600.600 degrees Fahrenheit. 600 degrees Celsius, or 1250 degrees Fahrenheit, at this moment.
Pay attention to where the bunny is melting.
It's liquefying right on that edge where the intensity is similar to the fire, which is somewhat creeping over top. - That is, in fact, Oh!Put the bunny down! Well, that's not a bad result. Not at all a bad outcome.
I would like to try some of this chocolate here.
How hot is it? It's warm. - Warm.
and delectable. as fondue.- Mm-hmm. That was awesome.
When the fluid CO2 has filled every one of the pores of the gel, now is the ideal time to make it supercritical.
The first time I saw a supercritical fluid, I would describe it as a spiritual experience.
We'll discuss that now.
I love how much you're into these autoclaves. - I adore aerogels.
Actually, we can heat this with a hairdryer to make a supercritical fluid.
As we approach the basic point, the outer layer of the fluid becomes sort of foggy.
Unusual, huh? Yes, those strange waves are there.
I'll speed it up so you can watch the surface vanish by and large.
The supercritical CO2 fluid that you are currently observing is aerogel, and in this state, the solid structure of the CO2 does not change.
Aerogel is pretty transparent, so it is almost impossible to see when viewed against a light background.
However, you can see that it has a slight bluish hue when viewed against a darker background.
In addition, according to Rayleigh scattering, all of the tiny nanoscale structures scatter light, which is why they are bluish in color, just as the sky is blue.
Furthermore, the force of light dissipated corresponds to 1 over frequency to the force of 4, and that implies it disperses more limited frequencies, similar to blue, substantially more than it dissipates yellow or red.
Furthermore, aerogel looks hazy in the bright and straightforward in the infrared.
Presently, what does this resemble, assuming I held it up to the blue sky ?What do you anticipate seeing? Would it appear extremely blue?
No, it looks yellow. This is due to the fact that the aerogel is actually scattering the blue light.
As a result, only longer wavelengths like yellow and orange reach our eyes.It has essentially the same effect as watching a sunset.
When you see the yellows and oranges of a sunset, the blue light has already been scattered by the atmosphere before reaching your eyes.
As a result, effectively viewing aerogel against a blue sky is comparable to viewing a portable sunset.
The aerogel's excellent thermal insulation is also due to its tiny pores. That's fantastic.
Does that look hot? It is certain to be hot.
You might think that because aerogel is mostly made of air—like 99 percent of air—it has the same thermal properties as air.
However, this is not the case.
In fact, it is a superior insulator to air.
This is because the pores are narrower than the average distance air molecules travel before colliding with something.
Their alleged mean-spirited path As a result, transferring heat to the aerogel's surface from the hot, swiftly moving air molecules below is extremely challenging.
The term for this is the Knudsen effect. It is so peculiar on the grounds that, you know, you don't expect something straightforward to impede the intensity that well, yet this truly does.
Aerogel insulation was utilized by NASA on the Sojourner, Spirit, Opportunity, and Curiosity rovers, and they intend to use it on subsequent missions to Mars.
For what reason does it require protection? NASA has also put aerogel to more unusual uses, most notably to catch dust from a comet as part of the Stardust mission because they don't want the electronics to get cold during the cold nights on Mars.
Since the aerogel is a very porous material with a very low density, the particles actually enter the aerogel when they hit it. As they travel through the aerogel, they basically break apart the network that makes up the aerogel, losing energy in the process and eventually stopping. The particles were travelling approximately six kilometers per second in relation to the aerogel.
This is really great for catching particles since, in the event that a molecule like that hits a strong surface, it simply stops, you know, right away.
It simply evaporates. also evaporates.
Consequently, should we anticipate seeing aerogel in our daily lives anytime soon?
Aerogel is used as thermal insulation when building skyscrapers in Antarctica, which is a running joke of mine.
For what reason do you say that? Indeed, in light of the fact that, at that point, they'll truly think often about how, exactly the way that it is effective on the grounds that it would be so cool there.- Right.
Therefore, instead of having ten feet of fiberglass insulation, you could use something like six inches of aerogel.
Researchers are at present dealing with diminishing expenses and expanding strength.
And that is accurate. They do have some stretch to them. Okay.
Better believe it, so there we go. So it isn't difficult to break.
Conclusion:
They have already made significant progress. Original silica aerogel, for instance, is hydrophilic.
About the Creator
Durga Prasad
My "spare" time is spent creating for myself and writing for others.





Comments
There are no comments for this story
Be the first to respond and start the conversation.