Amino acids are popularly known to be the building blocks of Protein. The concept it actually refers to is that, amino acid molecules combine together by a series of peptide bonds, to form a polypeptide. A chain of polypeptides is known as a protein.
In simple language, proteins are long chains of amino acids bind together with the help of peptide bonds. Here we will have a deeper look into the concept and try to understand what exactly is the chemical structure and how does glycine make an exception among all other amino acids.
Basics Of Amino Acids?
Amino acids are chemical bonds that are formed between two molecules when the carboxyl group (COOH) of one molecule reacts with the amino group (NH2) of the other molecule, releasing out a water molecule (H2O).
Let’s get a better understanding of these bindings in a broader sense.
There are 20 amino acids that are vital for organisms. These amino acids combine together to form different types of countless proteins. Proteins are further responsible for the smooth functioning of metabolic processes. The chemical structure of amino acids may explain the concept in a much better way.
As you can see above, amino acid is an organic molecule that is made up of a basic amino group (−NH2), an acidic carboxyl group (−COOH), and an organic R group (or side chain) which is unique to each amino acid. The term amino acid is short for α-amino [alpha-amino] carboxylic acid.
Every amino acid has this unique R group that differentiates between them. This structure basically defines their particular role in the further vital functions they are a part of. Still, interestingly Glycine manages to make itself unparalleled to the rest of amino acids.
What is Glycine?
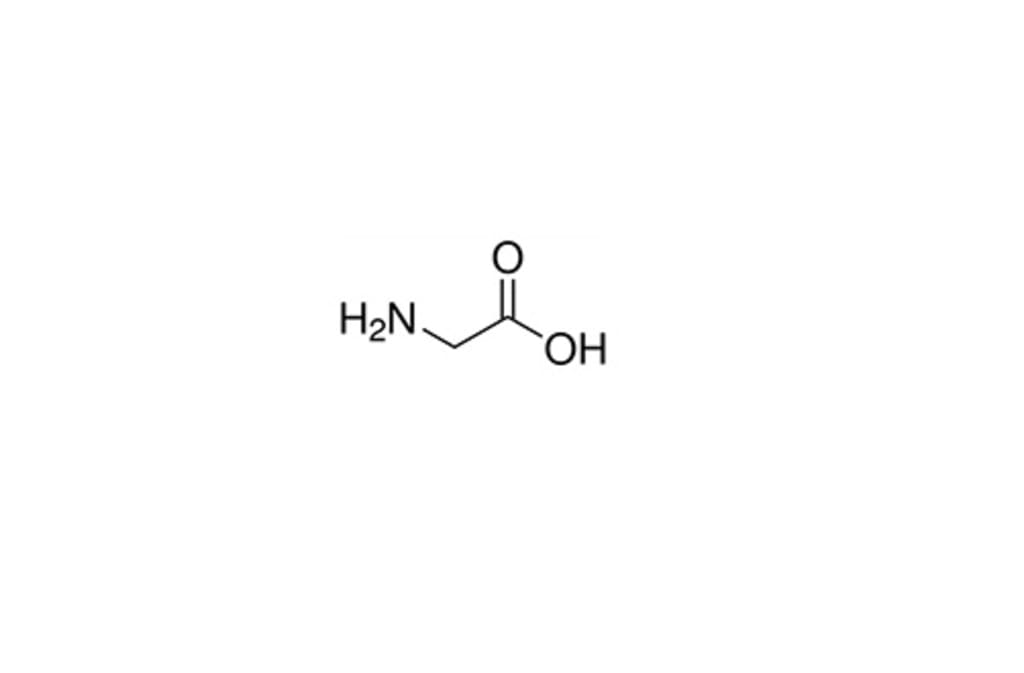
It is an amino acid having single hydrogen atom as its side chain. It is the simplest amino acid (as carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. It is one of the proteinogenic amino acids and is encoded by the specific codons starting with GG (GGU, GGC, GGA, GGG).
Glycine is essential to form the alpha-helices within the secondary protein structure, possible due to its compact form. Which is also why it is the most abundant amino acid in collagen triple-helices.
Glycine also works as an inhibitory neurotransmitter. It’s important to know that any interference with its release within the spinal cord can cause spastic paralysis.
Physical characteristics of Glycine include
• It is colorless
• It is sweet-tasting
• It’s a crystalline solid
• Only achiral proteinogenic amino acid.
• Can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.
Let’s have a look at the structure of glycine and see why is it having these exceptional qualities.
Benefits and Uses of Glycine
• they are needed to produce a powerful antioxidant
• it is a component of creatine
• it is the main amino acid in collagen
• it may protect your liver from alcohol-induced damage
• it may prevent heart diseases
• it may assist people with diabetes type 2
• it may help you against muscle loss.
• it is used in the synthesis of muscle proteins
• it is a precursor for a variety of important metabolites such as glutathione, porphyrins, purines, haem, and creatine.
• it acts as neurotransmitter in central nervous system and it has many roles such as antioxidant, anti-inflammatory, cryoprotective, and immunomodulatory in peripheral and nervous tissues.
• it is also involved in the production of other biochemicals that influence these body functions
• it regulates cognition, mood, appetite and digestion and the much needed glycine sleep
• it’s helpful in immune function and pain perception
Chemical structure of Glycine
The chemical structure of glycine is C₂H₅NO₂. As you can see it is an organic compound that contains 2 carbon atoms, 5 hydrogen atoms, 1 nitrogen atom, and 2 oxygen atoms. It is one of the 20 amino acids commonly found in animal proteins. It is one of the 20 amino acids commonly found in animal proteins.
Glycine is a major amino acid in mammals and other animals. Synthesized from serine, threonine, choline, and hydroxyproline via inter-organ metabolism it involves primarily the liver and kidneys.
Why is glycine an exception to other amino acids?
When we look into the basic structure of amino acids above, we notice two groups,
Amino group (NH2) and Carboxylic group(-COOH)
Both these groups are seen linked together with a carbon atom, known as alpha carbon.
This alpha carbon is also known as Chiral carbon.
A chiral carbon is a carbon atom having four groups bound to it. In amino acids, Amino, carboxylic, hydrogen atom and R group are the four groups bound to this carbon atom.
Now comes in picture is the hydrogen atom(H) and the unique side chain R group that is also bounded to this alpha carbon.
R is just to refer the side chain attached to these amino acids.
Glycine takes a different path at this stage. As, R group for glycine is just a hydrogen atom, rather than a carbon as is the case in all other amino acids, there is this duplication of atoms that take place. Hence glycine is the only amino acid that does not have a chiral carbon affecting its mirror image to be superposable on the original structure.
How does it affect?
This unique glycine structure helps it reside in parts of protein structures that are forbidden to all other amino acids.
Due to its small size, Glycine has an extreme conformation mobility helping it cause a bend in the chain. Thus, the steric hindrance around this bend is minimized. Its size is often critical in allowing polypeptide chains to make tight turns or to approach one another closely.
In simple terms it means, if the protein needs a bend, as in globular proteins, Pro or Gly will often be found. Thus, the alpha-helix is broken to bend, because Pro and Gly are thermodynamically destabilizing to alpha-helices. Among these pathways, GCS is the major enzyme to initiate glycine degradation to form ammonia and CO2 in animals.
Takeaway
As is evident Glycine plays an important role in various functions that are vital for human body to properly function, which is somewhat possible due to the specific chemical structure it owns.





Comments
There are no comments for this story
Be the first to respond and start the conversation.