Dr. Ganesh Suntharalingam was called in when his colleague explained the situation to him.
Rob: "They had the mask tightly on our faces because the blood and fluids were leaking into our lungs."
They were going into kidney failure, but there weren't enough machines, so police vans were carrying equipment from all around town.
Dr. Suntharalingam: "There was no rule book to deal with this kind of situation. Looking from the outside, they had unstable blood pressure, a problem with breathing, and their organs were failing."
Dr. Bradford: "I kept reading through the document and all the detail about the drug. There was an unlikely possibility of a cytokine storm; an extreme autoimmune reaction."
A cytokine is small proteins involved in the inflammatory response to injuries or to an external infection. Cytokine storm is when these proteins become extremely active. They start attacking their own cells.
Treatment for this included very strong immune-suppressants, a high dose of steroids, but the problem with this was the medicine reacting with the infection, worsening the condition of the participants.
Dr.Nicki Panoskaltsis, a hemato-oncologist was brought into the case.
"I got called at 12:04 AM and I didn't believe him, because I thought it was so incredulous to do this to eight healthy men."
"It was about 3:30 AM and my phone rang. It was one of those nurses. She said there had been an issue with the drug trial and I needed to get down," said Katrina Oakley, current wife, and fiancé at the time. "It was pretty scary to see someone you love to be so disfigured, who was just normal the day before."
Dr. Bradford: "In the morning, the story was on all the pages. Somebody had used the word "Elephant Man" when they saw the swollen faces of the patients. The fluids were given in and they were leaking out, so they had the swollen faces."
Scotland Yard became involved, because they wanted to know if there were any foul play involved. The police contacted the government body that regulates clinical trial—The MHRA—because they believed there was some tampering involved.
Katrina: "He was in and out of consciousness. He had enough strength to squeeze my hand, but that was about it."
The MHRA published a report on the identity and purity, and if there was any contamination of the drug, the reports were normal.
David: "After two days or so, that's when I felt things were improving."
Rob: "I remember feeling really hungry and that I was going to a safe place."
Dr. Nicki: "Just a fantastic example of how people come together when things get really tough."
Seven days since the trial began. Two patients were still critical, and the remaining four were removed from the ICU to an NHS ward.
David: "We had no immune system because it was entirely destroyed by the drug. We were advised not to take trains or buses, just in case somebody coughed and we had no way to fight it off."
Twenty one days into the trial, Ryan remained in critical care. When Rob visited him, he recalled that his fingers were blackened.
Rob: "There were no hopes of saving his fingers, and someone was responsible for it."
Ryan was in the hospital for four months. He suffered from pneumonia, septicemia, and dry gangrene. His fingertips fell off and his toes were amputated.
David married Katrina three months after the drug trial. He was worried about his chances of having kids.
The MHRA showed there was no tampering with the drug, and Gene Walter, the lawyer, said the reports by the MHRA were rushed out.
The reports concluded with:
"The adverse effects were the result of 'An unpredictable biological action of drugs on the human.'"
Dr. Bradford: "Everything we did, we followed protocol. If you knew what would happen, we wouldn't need to do it. That's why you do the trial."
In 2009, PAREXEL settled out of court and all six men received undisclosed amounts.
Aftermath
Scientists still can't explain why TGN1412 acted the way it did.
An independent study report into the drug made twenty-two recommendations to improve the safety of first-in-man trials.
One of the critical recommendations was that the individuals would be receiving the drug separately and not all at once. The other recommendation was the size of the dosage.
The report was accepted by the European Medical Agency and applies to the whole of Europe.
Dr. Bradford: "I participated in over 300 medical trials. That was the last medical trial I ever I did. I felt guilty every day for years. I'd like to look at them in the eye and apologize."
David didn't develop cancer and ended up having three kids with Katrina.
In 2013, TGN1412 successfully passed phase one human trials in Russia under the new name TAB08.
They used 0.1 percent of the antibody that was used in the London trial and it was infused into the bloodstream at least forty times slower.
TGN1412
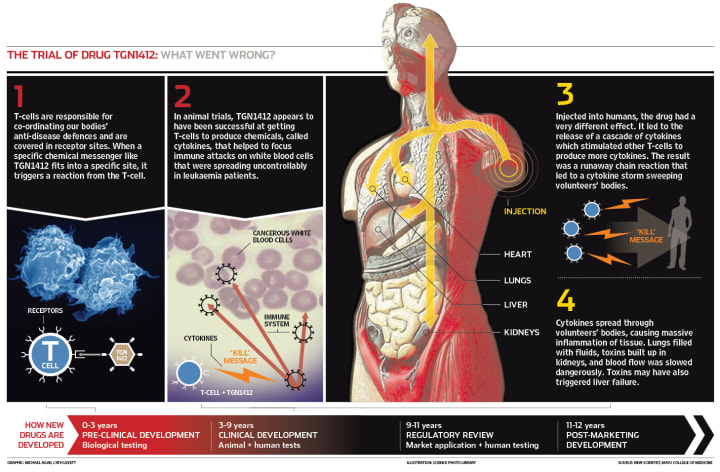
A simpler description of how TGN1412 failed in 2006.
About the Creator
Sarah Lee
Write about whatever catches your eye and gets your brain firing.






Comments
There are no comments for this story
Be the first to respond and start the conversation.