The Elements of Life
Ancient Alchemy Through the Lens of Modern Science

There is a fundamental question that arises as a consequence of that strange thing humans do where we know we exist. Namely, "what the heck is all this stuff made of?" Or more specifically, "what am I made of?" This most fundamental of inquiries has been pondered for about as long as there have been people around lucky enough to stave off disease, famine, and warfare. It's no simple question to answer, but today we are fortunate enough to live in a time when children can be handed the answers to these questions before they revolve around the Sun ten times. I've met and worked with more school groups than I can recall, and I'm always astonished at their ever growing capacity to learn. Even middle schoolers today understand the basic structure of atoms, something that has vexed the greatest thinkers throughout history.

If you have ever taken a basic chemistry course, then you were probably forced to memorize the periodic table of the elements. While it may have seemed tedious and confusing at the time, it's quite remarkable when one considers how far we've come over the millennia. The periodic table is one of the greatest crowning achievements of human thought, and it is arguably the most important chart in not only science, but all of history (perhaps only in competition with the standard model of particle physics). Rightfully, this gem of knowledge took centuries of meticulous experiment and observation to construct. It wasn't until the mid-nineteenth century that Dmitri Mendeleev was able to construct the table and begin to understand just what the basic building blocks of the universe (and our bodies) truly were. As it turns out, every one of the elements in the universe is merely a different combination of the same three ingredients: protons, electrons, and neutrons. This is quite remarkable, especially when considering how many diverse properties matter can have.
Before we understood the fundamental constituents of matter there were four main elements, at least in western thought. They are based on the philosophy of ancient Greece some two millennia ago, and are known today as earth, water, air, and fire. The Greeks based these elements on four principal qualities; hot, cold, dry and wet, which we might attribute in modern language to their energy density and composition. We now know of course that these ideas are but crude approximations for the true complexity of the universe. But even though these ideas may seem archaic today, the principles behind them still hold grains of truth. They corresponded with the four states of matter; solid, liquid, gas, and plasma. Not only that, but interestingly they are each harnessed within the human body in some capacity. Perhaps not in the crude sense that they were understood by the Greeks, but by analyzing these various phenomena through a modern scientific lens, one can see how harmonious nature truly is. So let's go through each element one by one, in the order from coldest to hottest, to see just what we're truly made of.
Earth

We begin with the coldest and firmest state of matter. It is the foundation on which we stand and grow. On our planet the ground harbors the oceans and the sky. Under the surface its molten churning spurs on the magnetic field that shields us from ionizing radiation. For ancients it symbolized prosperity, fertility, stability, abundance, nourishment, and dependability. They described it as a combination between cold and dry, which rather concisely describes matter at low temperatures. But we don't just need the Earth to stand on, we also use it for our own physical foundation, as well as nearly all of our core vital functions.
How it's used in the body:

Although you might not think about it, minerals are a crucial component of your diet. As in, if you don't eat some rocks, you'll die. Salt is a perfect example. Table salt is comprised of sodium atoms bonded with chlorine atoms (sometimes with iodine tossed in for processing). Separately, sodium is needed for the brain (more on that later), and chlorine is used as an essential ingredient in the hydrochloric acid our stomachs use to digest food. Chloride ions also move freely across the body to maintain osmotic pressure and the acid-base balance in our blood. This is why salt intake is so often linked with water retention.

Not only do we need rocks in our diet, our organism also utilizes a variety of metals as well. As but one example, iron is crucial for the hemoglobin in our blood to transport oxygen. Interestingly, iron is also the reason why our blood is red. However, many animals use other metals for their respiratory systems, which results in various different blood colors (e.g. nickel result in a blue hue). The human body uses all sorts of these types of elements for its basic functions, albeit in small quantities. Their work is also quite subtle, such as magnesium, which is a primary ingredient in epsom salts, and can soothe the body and help it repair itself. Magnesium alone is required for the production of over 300 enzymes vital for nerve and muscle growth, which is why epsom baths are perfect following any workout.
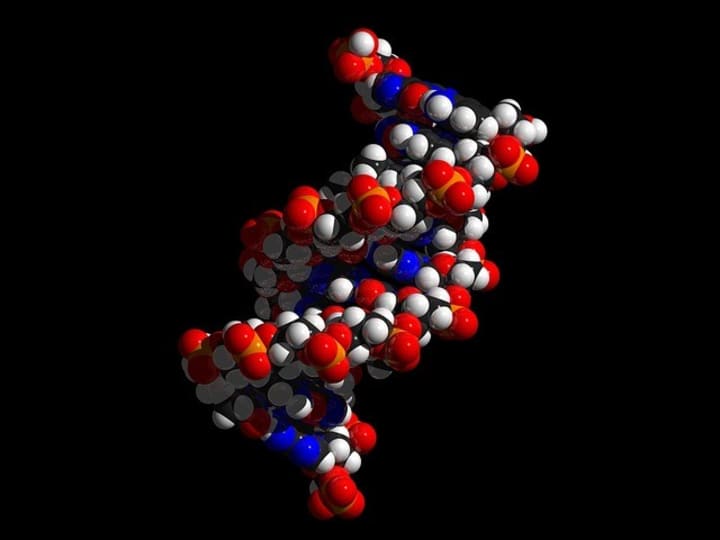
But one earth element stands above all others as the central building block of not just the human body, but as the bedrock of all known life: carbon. Even in our DNA (showed here), carbon is the lynchpin that holds organic molecules together. This is where the distinction between the four alchemic elements breaks down, as it isn't even completely accurate to consider carbon "earth", since it is found in the air as well. However, since in its pure form at room temperature it acts as a solid, and because it provides the base structure for solid living organisms, I would consider it close enough. Carbon's exalted status comes from its ability to make diverse bonds with nearly all other atoms. While many elements have a preference of gaining or losing electrons in order to be stable, carbon is perfectly balanced, making it the ideal atomic companion. It is also a relatively small atom, which helps it hold onto electrons even more.
But despite all the fascinating ways rocks and minerals interact with one another, none of it would be possible if they didn't have a fluid medium to travel through.
Water

It is the elixir of life. Covering over two-thirds of our planet's surface, it's no wonder water was considered so primordial to ancient cultures throughout history. The ancients often associated water with having cleansing power. Even for many today it is symbolic of healing, fluidity, purification, regeneration, stability, strength, and change. It symbolizes death as well as rebirth. It is life-giving, but can also be destructive. Fresh water stands for life and good health, while polluted/stagnant water is symbolic of bad health. The water element was described as the amalgam of cold and wet. While the ladder description may seem trivial, calling water "cold" actually has subtle inaccuracies in the context of the universe. The element most vital to life is actually quite rare in the form it is found in here at home. Unlike the earth element—which as traditionally described by the ancients includes dozens of periodic elements—water is among one of the most simple molecules, just one oxygen atom bonded with two hydrogen atoms in the shape of Mickey Mouse's head.

Furthermore, the elements that make up water are by and large some of the most abundant elements in the whole universe. The first two elements—hydrogen and helium, made up of one and two protons respectively—are so simple that they could actually form immediately after the big bang 13.8 billion years ago. As a matter of fact, they comprise the vast majority of visible matter, hydrogen making up three-fourths and helium making up nearly one quarter. But directly after helium is, as you could probably guess, oxygen. So it's quite likely that water is one of, if not the most abundant molecules in the universe. The thing is, space is pretty cold, and if it isn't near absolute zero, then it's thousands of degrees nearby a star. Water is only liquid within a narrow range of temperatures—from 0°C to 100°C—which ironically makes it quite rare. In order for a planet to sustain any type of ocean, it needs to be the right distance from its star, and needs to have the right combination of mass to hold onto it and an atmosphere. But a water world also needs more subtle features, like spin to generate a magnetic field. The fact that our planet is tilted slightly by 23.5° also helps a little, as it allows for the occurrence of seasons which spread the Sun's heat over the surface of the planet through the course of its orbit. Add in the fact that virtually all of it is either too salty to drink or is frozen at the poles (for now), and this makes liquid water in many ways more valuable than gold.
How it's used in the body:
Well over two-thirds of the human body is comprised of water. To approximation, the human body is essentially a conglomerate of fatty sacks of water filled with salt. The evolution of life can basically be summarized as the story of water putting on some clothes of carbon and walking onto dry land until eventually it turned into hairless apes that write articles on social media platforms. But to truly understand what makes water so amazing, one must understand it at the most basic molecular level. Due to quantum effects (which I explain thoroughly in the second chapter of my book) water is a polar molecule, with a slight positive charge on one end and a negative charge on the other. This is not only crucial for dissolving molecules so they can be used in the body, but it is also vital in that it allows the body to store non-polar molecules which don't dissolve in water. The best example of this behavior can be seen in fats, which store energy in the body and make up the outer membrane of all cells. To add to water's amazing qualities, since oxygen is so selfish with electrons water frequently loses and gains additional hydrogen atoms. These loose hydrogen atoms are what make substances either acidic (with excess free hydrogen) or basic (with a deficit of hydrogen). Since water has a pH of 7, neither acidic or basic, this molecular quality allows water to act as either an acid or a base in the presence of more extreme molecules, thus making it even more ideal for chemical reactions. Unlike nearly all other substances, water also expands when it freezes, which means that ice rises, allowing life to survive even when oceans are frozen over, which has happened several times in Earth's history. Thanks to all of these traits, water is a perfect medium for the complex chemical reactions that sustain life.
Now that we have a body with a foundation and a fluid, it's time to drive some chemical reactions so that our bodies can actually keep moving!
Air

Although we don't often think about it, we live everyday waddling through a deep sea of fluid. It only so happens that this fluid is much thinner than our bodies. But whether we notice or not, we rely heavily on the thin ocean of molecules that fills the air. As it is the medium through which we speak, air symbolizes communication, and therefore intelligence, perception, knowledge, learning, imagination, creativity, harmony and travel. This source of life can also become a force of terrible destruction, as seen through tornadoes and hurricanes. Interestingly, air was described as being hot and wet by the Greeks. This might sound strange at first, but the air we breathe truly is "hot", at least relative to what it would be if it was solid. You might recognize frozen carbon dioxide for example. We call it dry ice, and it is so cold that it can actually burn you. On an even greater extreme, when the nitrogen that dominates our air freezes, it's a good sign that you're out in the vacuum of space far far away from any source of heat like a star. My favorite example of this is Neptune's moon Triton, whose surface is composed primarily of nitrogen. I would always bring up Triton during my talks on the volcanoes in the solar system, as it is one of the few locations besides Earth that has cryovolcanoes thanks to a greenhouse effect on Triton's surface. So while it might not seem like it in the midst of winter, there's no denying how toasty our atmosphere is relative to what it could be. Couple this with the fact that our air is filled with water vapor, and the hot and wet moniker actually fits quite nicely.
How it's used in the body:
Though the human body is primarily solid, we would be nothing more than inert soup without our gaseous lungs. With them we soak up the air to take what we need to invigorate our bodies. Comically enough, the majority of the air is actually useless to us. Nearly four fifths of it is comprised of nitrogen. Of course, nitrogen is crucial for the survival of life. It is used to form the amino acids that build all sorts of structures in the body, including not just our muscles, but our very DNA. The problem is the way it is bonded in the air. When nitrogen atoms pair together, they form ridiculously strong triple bonds, sharing three electrons between two atoms. This renders them nearly inseparable and ergo useless to any living organism, with the exception of some primitive single celled bacteria. This is why plants need fertilizers and why we need to eat in order to get the nitrogen required to survive. Only one fifth of the air is useful to us, but it goes a long way.
Animals and plants have a wonderfully symbiotic relationship revolving around the use of oxygen. Normally, oxygen is a voracious element that readily soaks up electrons and bonds to other molecules. The high reactivity of oxygen is so paramount to the evolution and history of life, that I spent a large portion of the beginning of my book just discussing oxygen and its unique quantum behavior. For this reason, oxygen is seldom found "free" like it is on Earth, bonded only with itself. It is only thanks to the magic of photosynthesis that the waste products of plants are available for animals to breathe and use to grow. Thanks to its high reactivity, oxygen can be found mixed with nearly every organic chemical in the body to some extent. Perhaps most importantly, it drives the aerobic respiration that converts the chemical energy in our food in order to fuel our cells. Fun fact, this means that breathing is the single most effective way to lose weight. After all, the oxygen you breathe doesn't itself go into making anything in the body, it just goes in and comes back out with a carbon molecule attached (as CO2). Where do you think that carbon comes from? The only source is your body. This means that every breath you take is a free way to lose weight. All you have to do is inhale, and your body naturally oxidizes. And that leads us to the next element.
Fire

Fire was considered to be the first element to be born when the universe was born. Fire is attributed with transformational and purifying powers. It can give warmth, and it can also incinerate. To some, the element symbolizes incredible energy, activity, creativity, passion, freedom, power, love, anger, will, and courage. In many ways this view is accurate, as the universe started out hot and dense, although it was far far hotter than any flame here on Earth, or any star for that matter. It is also fairly accurate because what we see as fire was also one of the first forms energy took after the big bang: light. You see (get it?), unlike what many ancients may have thought, fire itself isn't a substance. It isn't something that can be held or properly contained. Instead, fire is a process. Those who have been trained in wilderness survival may remember learning about fire with the use of a triangle, with each of the necessary components on each of its vertices. Three ingredients are required for pyrotechnics: heat, fuel, and oxygen. What they don't teach you in survival courses is that these three ingredients are required because fire is a chemical phenomena involving the rapid oxidation of a substance, usually carbon. Light is nothing more than electromagnetic waves, and it communicates the electromagnetic force between electrically charged objects, like molecules and the atoms that make them up. So when you see fire, what you are really witnessing is the macroscopic amalgam of light produced by trillions of chemical reactions between molecules.
How it's used in the body:
You might think it quite dangerous for our bodies to be near fire. But once you realize that fire is merely a chemical reaction that emits light, it becomes quite clear how virtually everything we do involves what the ancients might call "fire". And there's a good reason fire feels hot to us, even though relative to a star fire is a thousand times colder. The key is the type of light fire emits.

Since light is just the communication of electromagnetic charge, everything that contains atoms produces light. EVERYTHING. The light produced by the random motion of atoms inside of objects is known as blackbody radiation, and everything we see is therefore a blackbody. In addition, every blackbody releases all wavelengths of light, from gamma rays to radio waves. However, objects don't emit all wavelengths equally. Instead, they emit light along a curve that peaks at different wavelengths relative to the temperature of the object in question. My favorite example is the Sun, which at a surface temperature of around 6,000°C peaks at the green wavelength of light, which is precisely why our eyes focus on that wavelength.

Humans also glow! But at the frigid temperature we live at, the blackbody curve of our bodies peaks at a much less energetic wavelength. Specifically, our bodies glow in the infrared (just less energetic than red light). One can even see this with the proper camera. This is why we interpret infrared light as heat. Fascinatingly, the reason behind this has to due with the size of the molecules that make up our bodies. Many of our favorite chemicals—from carbon dioxide to water—are potent greenhouse gasses, because the wobbling of molecules that size releases and absorbs infrared light. This brings us full circle back to the environment in which we live, and to our climate, which harbors the last key element of life.
Electricity

Finally, we come to the fourth state of matter. When solids melt, liquids evaporate, and gasses are bombarded with electromagnetic radiation, the structure of atoms breaks down, charges run free, and matter turns into plasma. Technically, electricity is fundamental to all the other elements of nature. Not only are electrons one of three parts of all atoms, they also communicate photons by virtue of having electric charge, hence why lightning strikes are so luminous. For many ancient cultures, lighting was poorly understood. Often times, because a lightning strike would ignite nearby trees and houses (before the days of lightning rods, thank you Ben Franklin), lightning would we associated with fire itself. When you read about descriptions of fire raining down from the heavens (like in The Bible), you're seeing lightning through the primitive eyes of our ancestors. But just because they didn't know what it was, doesn't mean they didn't know to respect its power. Look toward virtually any mythology around the world, and you will find sky deities near the top of the divine pantheon. Zeus and Thor provide just two prominent examples of this, and of course the Hebrews always blamed their god for bad weather in the Old Testament.
Although it should come as no wonder why it took humans so many millennia to understand the nature of electricity. Negatively charged electrons are naturally attracted to the positively charged protons within atomic nuclei, and dislodging them is no easy task. There's good reason why lightning has been both revered and feared as punishment from the gods. Remember those tight nitrogen bonds that pervade the air? It takes a potential gradient of over 100 million volts to split them apart and produce a lightning strike. When one occurs, it heats the air to over 20,000°C, putting out a trillion watts of power and 30,000 amps of current, enough to power the whole United States long enough for you to read this article. Thankfully, metals have a much looser grip on their electrons, and therefore it requires far less energy to move them through our homes and appliances. But that doesn't mean we don't still need to respect its power, because electricity is what allows us to move as well.
How it's used in the body:

Arguably the most important and remarkable feature of our bodies is our nervous system. Without it we would essentially be on par with plants and fungi. Although that's not necessarily a bad thing, all they have to do is drift through the air and grow somewhere, soaking up sunlight or dead matter. They don't have to worry about finding a job, taking exams, or contemplating the nature of their existence. But they also can't run away from things that want to eat them, nor do they have the capacity to do the one thing that all life will eventually need to do in order to continue surviving: leave the Earth. The hundreds of neurons in our body not only allow rapid communication between all of our other vital organs, they also allow us to perform such complex behaviors as speaking and and mathematics. Not to mention they allow us to do the whole thinking thing, that weird phenomena were we know we exist. And it's all thanks to electricity. However, it should be noted that the electrical signals in the brain aren't quite like the those found in computers and electronics. While appliances move individual electrons through the outer fringes of metal atoms, our nerves communicate by transporting ions, with protons and electrons intact. Specifically, they rely on what is known as the sodium potassium pump. Thanks to negatively charged proteins within the nerves, these positive ions are able to flow from one nerve to another, thus allowing everything we think, feel, and do.

Through our nerves we can now finally see the glorious symphony of nature that goes into human life. From the earth that makes up the bodies of our cells, to the fluid that fills them, to the electromagnetic reactions that drive their chemical processes, it takes the majority of the periodic table to create a healthy human being. Together, these elements have coalesced from cosmic dust, forming into us, a mirror through which the universe can understand itself.






Comments
There are no comments for this story
Be the first to respond and start the conversation.