Solvent Extraction
Introduction, principle, procedure, types and its applications.
SOLVENT EXTRACTION
INTRODUCTION
It is a method for dividing things. To remove a substance from a mixture, it is said to be dissolved in another immiscible solvent. Solvent extraction is exemplified by the process of separating benzene from naphtha using sulfolane as a solvent. Another way of separation is distillation. Through the use of a more soluble, immiscible solvent, a component is partially removed from a solution or mixture.
PRINCIPLE
Solvent extraction works on a very simple principle. The purpose is to dissolve (solvate) a target molecule or collection of chemicals (solute) in a liquid (solvent) and wash them out of solid plant material. After that, the solvent is separated from the solute.
Solvent extraction, commonly referred to as liquid-liquid extraction or partitioning, is a method for separating and purifying mixture components depending on how differently they are soluble in two immiscible solvents. The components disperse themselves between the two solvents according to their relative solubilities, which is based on the partitioning principle.
PROCEDURE
Solvent extraction, commonly referred to as liquid-liquid extraction or partitioning, is a multi-step process. Here is a general description of what happens:
Choosing Solvents: Select two solvents that are immiscible and appropriate for the components that will be extracted. Typically, there are two solvents: one aqueous (such as water) and one organic (such as dichloromethane or ethyl acetate). The components' solubility characteristics and compatibility with the solvents determine which solvents should be used.
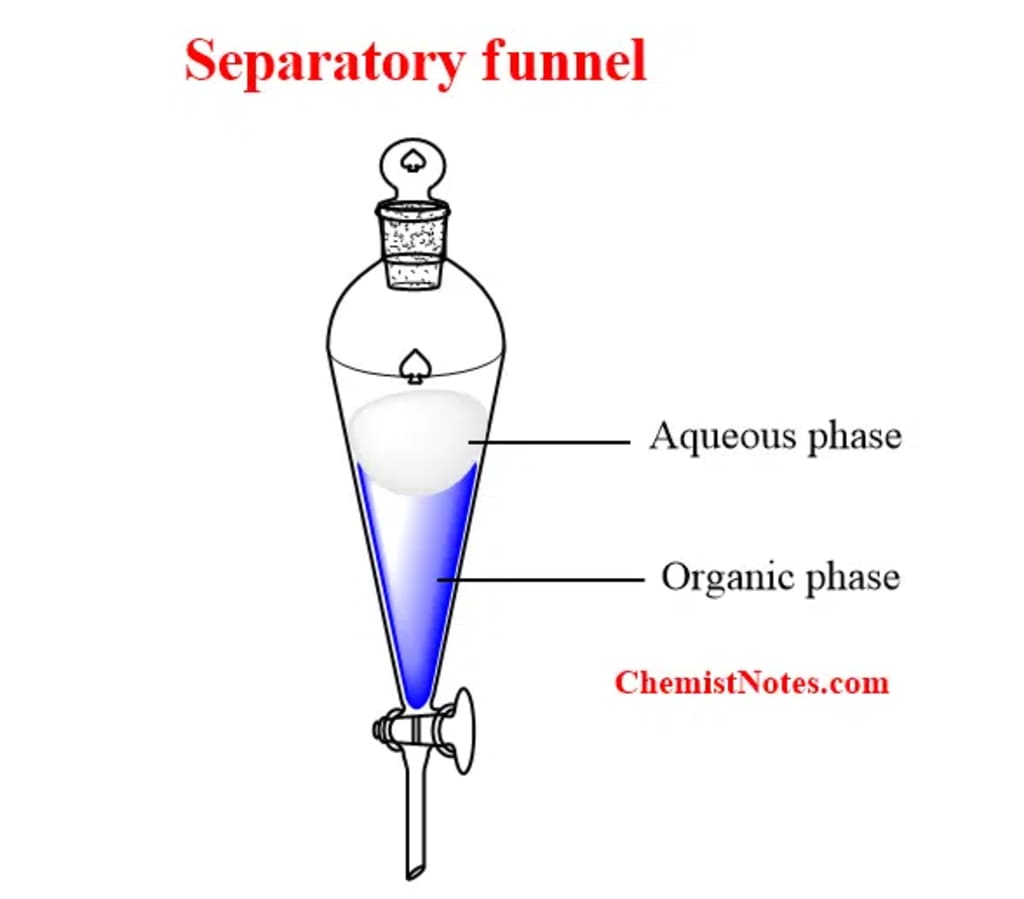
Preparation of the Mixture: In an appropriate container, such as a separatory funnel or a centrifuge tube, combine the mixture comprising the components to be extracted with the two immiscible solvents. The combination could be a suspension, solution, or emulsion.
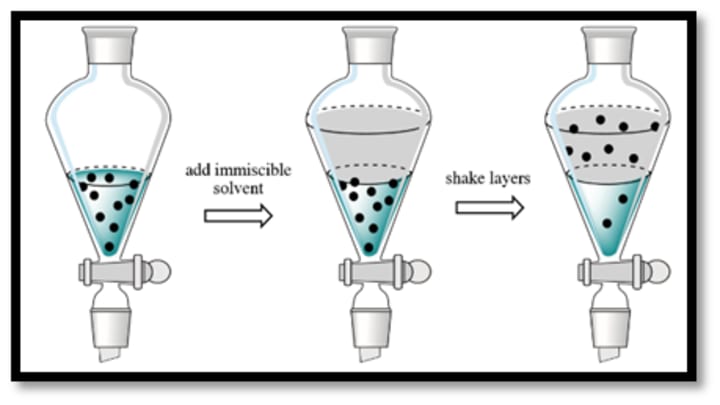
Mixing and Phase Separation: Shake, swirl, or vortex the liquid to thoroughly combine the solvents. The components can now divide between the two solvents as a result. Allow the mixture to settle after mixing to facilitate phase separation. The separation may happen naturally or centrifugation or gravity settling may be required, depending on the solvents employed and the characteristics of the components.
Phase Separation and Collection: Carefully separate the two solvent layers after the phases have separated into discrete layers. Decantation (pouring off one layer while keeping the other), centrifugation, or the use of a separatory funnel can all be used to accomplish this. The solvent layer containing the desired components should be collected. If more processing is required, the other solvent layer can be discarded.
Recovery of Extracted Components: You can use rotary evaporators or other suitable techniques to evaporate the solvent at reduced pressure in order to recover the extracted components from the solvent layer. The extracted components of interest are contained in the leftover solid or liquid residue.
TYPES
Following are the types of solvent extraction, based on the medium or phases of the substances extracted or separated;
Liquid–liquid extraction.
Solid-phase extraction.
Acid-base extraction.
Supercritical fluid extraction.
Ultrasound-assisted extraction.
Heat reflux extraction.
Mechanochemical-assisted extraction.
Maceration
APPLICATION
Many different applications make substantial use of solvent extraction, including:
1. Natural product extraction: Plants, herbs, and flowers are examples of sources of useful substances that can be extracted using solvents. It enables the separation of flavourings, perfumes, and essential oils as well as active medicinal components.
2. Metal Extraction: The mining and metallurgical industries frequently use solvent extraction to extract and purify metals from ores and concentrates. It makes it easier to separate and extract metals including cobalt, uranium, copper, nickel, and rare earth elements.
3. Environmental analysis: To extract and concentrate contaminants, such as heavy metals and organic compounds, from soil, water, and air samples for analysis and detection, solvent extraction is employed in environmental monitoring.
4-Pharmaceutical Industry: Pharmaceutical chemicals and intermediates are isolated and purified via solvent extraction. It facilitates impurity removal, isomer separation, and active ingredient concentration.
5- Nuclear Industry: The extraction of radioactive elements like uranium and plutonium from spent nuclear fuel or ores requires the use of solvents, which is essential to the nuclear fuel cycle.
Analytical chemistry: The preconcentration and cleanup of samples before to analysis are done using the sample preparation technique known as solvent extraction. It makes it possible to separate and enrich target analytes from intricate matrices.
Utilizing their solubilities in various solvents, components can be efficiently separated and purified using the versatile technique of solvent extraction. It is widely used for a variety of applications in many different sectors and scientific fields.
The selection of solvents, the pH of the aqueous phase, the temperature, the agitation, and the presence of additional chemicals that may compete for solubility are just a few of the variables that might affect the selectivity and effectiveness of solvent extraction.
In conclusion, for the purpose of separating and purifying components from mixtures based on their various solubilities in two immiscible solvents, solvent extraction is a flexible and frequently employed approach. The components can disperse themselves across the two solvents thanks to the partitioning principle, which leads to their selective extraction into one of the solvent layers. This method is used in a variety of scientific fields and sectors, such as pharmaceuticals, natural products, environmental analysis,
About the Creator
Enjoyed the story? Support the Creator.
Subscribe for free to receive all their stories in your feed. You could also pledge your support or give them a one-off tip, letting them know you appreciate their work.




Comments
There are no comments for this story
Be the first to respond and start the conversation.