Phosphorus
Sources, Uses ,daily requirements and its role on health and environment.

Phosphorus
Introduction:
A macronutrient that is needed in substantial amounts is phosphorus. It's non-metallic. While at room temperature, it is colourless.
Glow in the dark
Phosphorous's characteristics
One of the three macronutrients necessary for plant growth is phosphorus. It is necessary for the procedure of photosynthesis, which transforms the sun's energy into food for the plant. It is also necessary for the growth of robust roots. For a healthy growing cycle, phosphorus must be accessible to a plant.
Sources of phosphorus:

The Uses of Phosphorous
Concentrated phosphoric acids are a component of fertilizers used in farming and agriculture. Phosphates are used for pyrotechnics, insecticides, toothpaste, detergents, special glasses, sodium lamps, steel manufacture, military applications (incendiary bombs, smoke screenings, etc.), and other things.
Daily requirements:
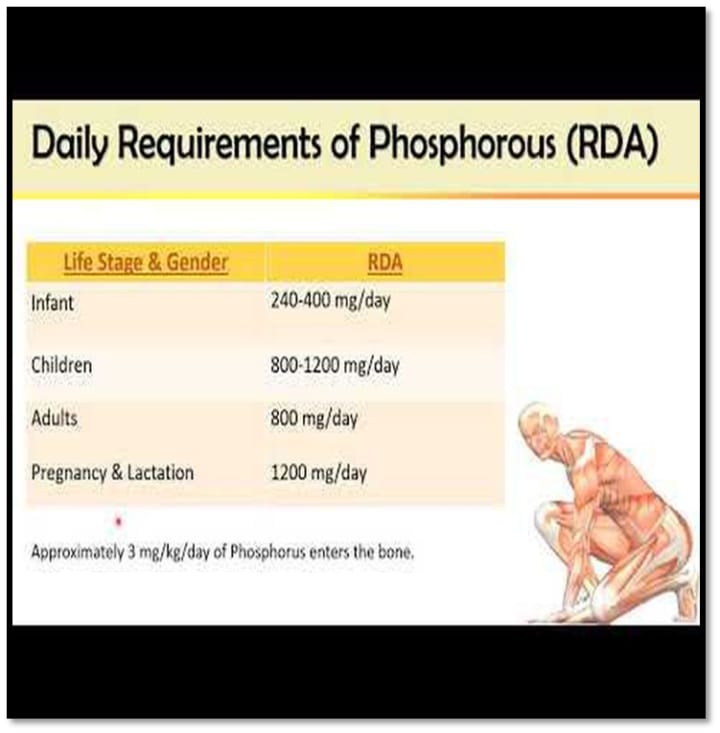
Effects of phosphorus on health
The most prevalent form of phosphorus in the environment is phosphate. Phosphates play a crucial role in energy transfer and are a component of DNA, making them important compounds in the human body. Phosphates are frequently present in plants as well.
The recommended daily intake of phosphate is 800 mg, although a typical diet only offers 1000–2000 mg per day, depending on how much phosphate-rich food is consumed.
Through the use of phosphate-containing detergents and the application of phosphate-rich manures to the soil, humans have significantly altered the natural phosphate supply. Additionally, phosphorus was added to a variety of meals, including cheese, sausages, and hams.
Phosphorus overdose can result in kidney damage and osteoporosis, among other health issues. Lacks of phosphate might also happen. These are brought on by heavy medication use. Health concerns might result from low phosphate levels.
White is the hue of pure phosphorus. The most hazardous type of phosphorus that we are aware of is white phosphorus. When white phosphorus appears in the natural world, our health may be seriously endangered. White phosphorus is incredibly dangerous, and exposure to it is frequently fatal.
Most often, those who died from exposure to white phosphorus did so after unintentionally ingesting rat poison. People who have been exposed to white phosphorus frequently experience nausea, stomach cramps, and tiredness before they pass away.
Skin burns can be caused by white phosphorus. White phosphorus when burned can harm the kidneys, the heart, and the liver.
Effects of phosphorus on the environment:
White phosphorus
When businesses turn white phosphorous into other chemicals or when the military utilizes it in munitions, it leaks into the environment. White phosphorus enters surface waters close to the factories that use it through wastewater discharge.
Given how quickly white phosphorus reacts with oxygen, it is unlikely to spread. When phosphorus enters the atmosphere via exhausts, it typically reacts with oxygen right away to create less dangerous particles. However, phosphorus particles in the air might have a barrier that stops chemical processes.
White phosphorus does not interact with other particles in water very quickly, therefore it will build up in the bodies of aquatic species. Phosphorus in soil will persist for a few days before it is changed into less dangerous compounds. However, phosphorus can linger for up to a thousand years in deep soils and at the bottom of rivers and lakes.
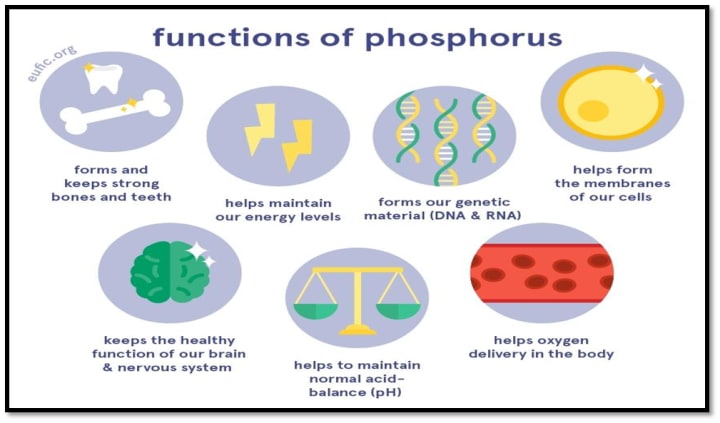
Phosphorus's function in human diet and health
There are two ways to get phosphorus: as inorganic phosphate or as organic phosphate. The primary supply of phosphate in diets made from plants is organic phosphorus, however inorganic phosphate additions are more bioavailable. Phosphorus is a crucial component of nutrition and plays important roles in the human body. It is essential for mineralization and bone development. Phosphorus is one of the key elements in the upkeep of bone health, as demonstrated by bench and clinical studies. A lack of phosphate causes bone pathology and clinical illnesses including rickets and osteomalacia. The mineral phosphorus is found in bones.
Teeth, DNA, RNA, ATP, a component of cell membrane, gene transcription regulation, enzyme activation, control of extracellular and intracellular fluid pH.Calcium and phosphorus make up roughly 80–90% of the mineral content of bone, with the skeleton holding 85% of the phosphorus. Intake of phosphorus must be adequate for the mineralization of the skeleton.
Deficiency of Phosphorus:
Bone discomfort, anxiety, fatigue, irregular breathing, joint stiffness, numbness, weakness, change in body weight, and issues with tooth development are all symptoms of a phosphorus deficiency.





Comments (2)
Wow. this is what I wanna find. https://fnafgames.io Big thanks!
Thanks for the information!