One of the types of alloys used in precision casting and casting modeling is ductile iron or ductile iron, which in this article we want to explain a little more about it and get acquainted with it.
History of Ductile Iron
Another type of cast iron is ductile iron, which was discovered in the 1950s. This type of cast iron is sometimes called "spherical cast iron" and in the UK it is called "SG cast iron" or "cast iron with spherical graphite". Ductile iron expanded for a decade until 1950, growing nine times, followed by a rapid increase in commercial order.
Properties of ductile iron
Because graphite appears in spherical ductile iron, there is an unusual mixture of different properties in this cast iron. The type, especially solidification, which produces spherical graphite is obtained by adding a very small but certain amount of magnesium to molten cast iron. The cast iron melt used has a composition similar to gray cast iron, only the amount of additional elements in it is limited enough.
Metallurgy The problem of adding magnesium to the melt is really complicated because magnesium is a very active metal and it evaporates at the temperature at which cast iron is molten. According to the above, in order to accurately control the process of production of ductile iron, methods have been invented and developed.
The high carbon and silicon content of ductile iron casts the advantages of the casting process and the excellent machinability of gray cast iron, but graphite spheres have little effect on the surrounding metal properties. Ductile iron has a high modulus of elasticity and our relationship between stress and relative length change is linear, its yield strength is in a very good range and, as its name implies, it also has good flexibility.
Various casting parts in a wide range in terms of size and design; They are cast with ductile iron.
Effect of chemical composition on the properties of unbreakable cast iron
All the elements that dissolve in ferrite strengthen it, but have different effects on increasing strength and hardness.
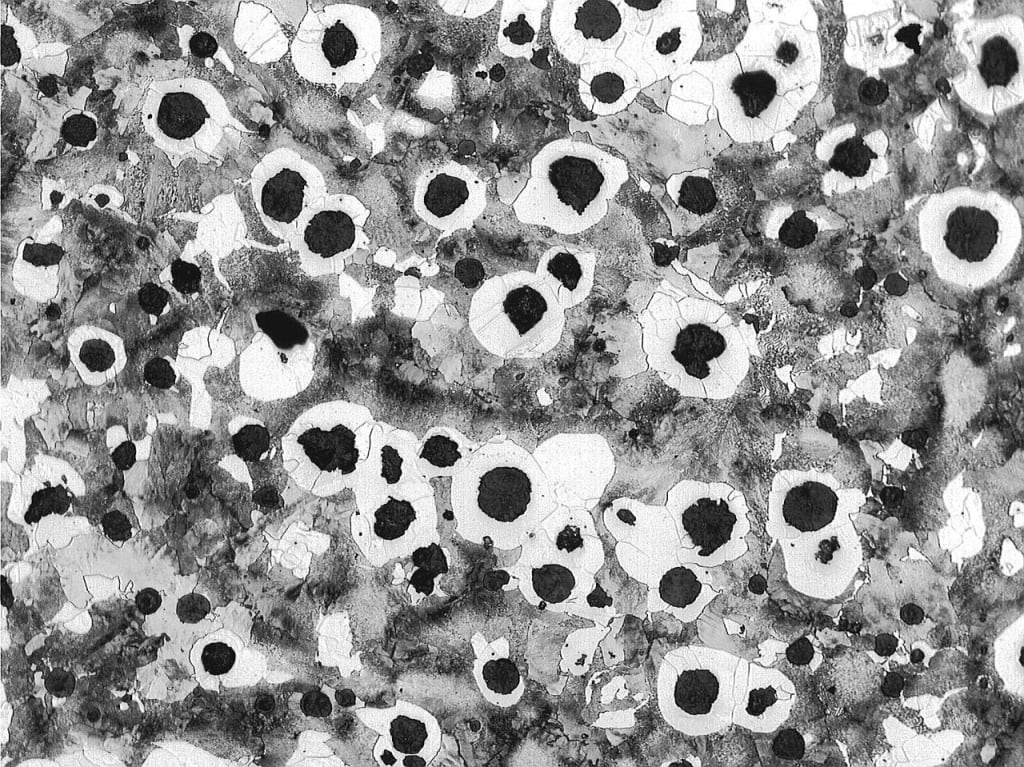
Carbon: The carbon content of unbreakable cast iron varies between 3-4%, but a more limited range of 3.6-3.9% is also common. More carbon is needed to make high-density spherical Graphites than gray cast iron. If the carbon equivalent is too high (for example, more than 4.6%), carbon may float (Figure below).

Unbreakable cast iron
Silicon: The amount of unbreakable cast iron silicon varies between 1.8-2.8%, but a smaller range of 2.2-2.7% is also common. Silicon affects the carbon equivalent, so as the amount of silicon increases, so does the number of spheres. The low amount of silicon in unbreakable cast iron increases the tendency to refrigerate; if the amount of silicon is too low, additional carbides may form in the thin sections. Silicon strengthens ferrite in unbreakable cast iron, but care must be taken to increase the temperature of soft-to-brittle fracture transfer.
Sulfur. The amount of sulfur in unbreakable cast iron is usually limited to less than 0.03%. Increasing the amount of sulfur means that more magnesium must be added to Spherify the Graphites. The sulfur content after Sphericalization is about 0.015%.
Phosphorus. Phosphorus has a strong effect on ferrite strength, but due to the harmful effects of this element, especially the inverse effect on impact properties and ductility, the maximum amount of phosphorus in the production of unbreakable cast iron is about 0.10%, but is usually limited to less than 0.05%. If the amount of this element exceeds the above limit, it causes the effect of separation phenomenon in the boundary area between the grains and the formation of brittle Steadyte eutectic structure. Phosphorus separation is quite evident in the intergranular boundary region in thick castings.
From a structural point of view, increasing the amount of phosphorus increases the amount of perlite and also increases the tensile strength and stiffness and reduces the amount of relative elongation, resulting in brittleness of the part.
Copper: Copper increases the strength of ferrite, but for this purpose is not used in the production of unbreakable cast iron because copper is a perlite-producing element and its presence makes cast iron more sensitive to harmful minor elements.
Nickel: can increase the strength of ferrite without the disadvantages of other elements, and therefore only silicon-nickel alloy can be used if the production of brittle ferrite cast iron is intended.
Other elements. Elements such as lead, titanium, aluminum, antimony, and zirconium must be carefully controlled because these elements encourage the formation of filamentous Graphites. Other elements that allow the formation of perlite or iron carbide, such as arsenic, boron, cream, tin and vanadium, should also be controlled.
The effect of alloying elements on perlite is a combination of their effects on ferrite, cement or carbide in addition to the effect of these elements on the eutectoid temperature.
Nickel, silicon and manganese are the main elements used in the production of brittle perlite cast iron. Nickel and silicon strengthen the ferrite and manganese sheets to form cementite sheets in perlite and thus the perlite structure is created. Nickel and manganese are effective in reducing the conversion temperature of austenite to perlite, which reduces the stability of perlite.
These cast irons have good abrasion resistance and are used to produce liner plates for some parts of the cement mill and parts of the crusher. Copper and tin can be used for perlite generation.
The effect of alloying elements on carbide is of special importance because the size and diffusion of carbide has a great effect on the properties of unbreakable cast iron. Silicon and nickel do not tend to carbidize and are graphitic. Manganese is a weak carbide and is mostly soluble in iron carbide. In the microscopic structure, manganese-induced carbide appears as cementite sheets in perlite. Chromium, molybdenum and vanadium are strong carbide elements and their solubility in cementite is limited. Chromium carbide particles are relatively coarse and hard and are harmful to the strength of the part and make them brittle. In some cases, the presence of 0.15% chromium has a very definite effect on the brittleness of the part. Molybdenum carbide particles are smaller, especially if nickel is present. Due to the small size and dispersion of molybdenum carbide particles, its effect on toughness is limited. Vanadium also has little use in the production of unbreakable cast iron because it is a strong carbide element. Because it causes the formation of coarse carbides at the grain boundaries, which in turn reduces the toughness and increases the brittleness of the part.
About the Creator
PGM
I do Casting , and I love Investment Casting Technology.






Comments
There are no comments for this story
Be the first to respond and start the conversation.