What is Inert Gas
A short description on inert gas
Introduction:-
Inert gases, also known as noble gases, have long captured the fascination of scientists and chemists due to their unique properties and relatively scarce presence in the Earth's atmosphere.These gases are placed to Group 18 of the periodic table and include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Due to their relatively low abundance, inert gases play essential roles in various scientific, industrial, and everyday applications. In this article, I will try to explain the meaning What is Inert Gas, their properties and applications of inert gases, shedding light on their significance in different fields which is a very interesting topic of school going students of class (IX) and class (X)
Explanation:-
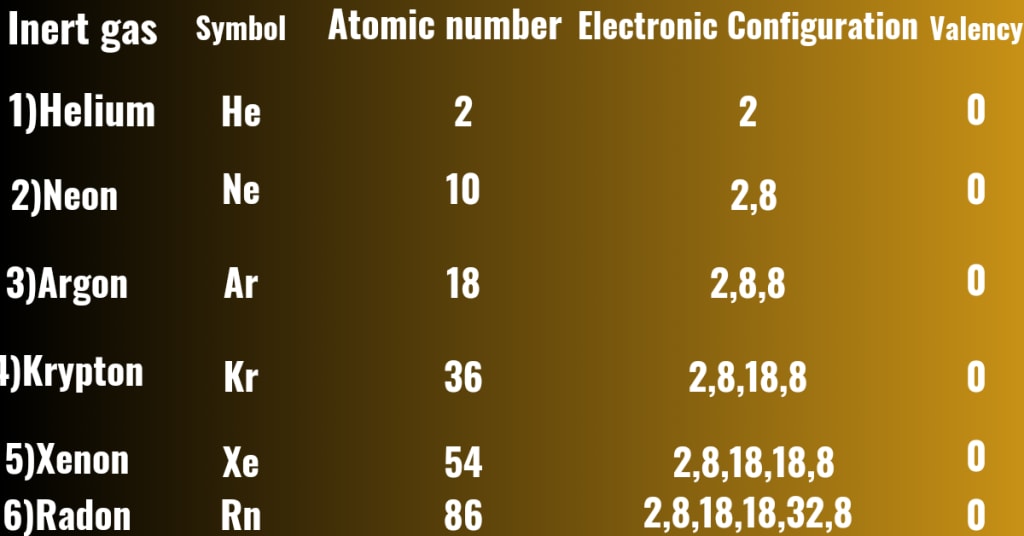
The elements whose valence shells are full filled with 2 (In case of Helium) or 8 electrons (In case of Neon,Argon,Xenon,Radon,Krypton) ,they are called valence shell full fill elements, so they have no tendency to accept or give up electrons in chemical reactions , they are chemically inactive elements and are called noble gases.
The name of all inert gases,their atomic numbers,electronic configuration and their valencies are shown below :-

What is Inert Gase' actually Noble gases, also known as inert gases, are a group of elements located in the far right side of the periodic table. These gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). In another way,we can explain them as "inert " gases because of their relatively low chemical reactivity - they were believed to be completely inert until the 1960s when their reactivity was discovered.
Properties of Inert Gases:-
1.Chemical Inertness: The term "inert" in inert gases stems from their remarkable lack of reactivity. This property arises due to their stable electron configurations. Noble gases have full electron shells, rendering them chemically stable and unlikely to form compounds with other elements. This makes them stand out from other elements in the periodic table, which typically engage in chemical reactions to get stable electron configurations.
2.Colorless and Odorless: Inert gases are typically colorless and odorless, making them hard to detect with our senses. This characteristic has both practical and aesthetic applications. For instance,Neon, is famous for its use in neon signs due to its vivid red-orange glow when subjected to electrical discharge.
3.Low Boiling and Melting Points: Inert gases have exceptionally low boiling and melting points compared to most other elements in the periodic table. Helium, for instance, remains in a liquid state even at extremely low temperatures, just a few degrees above absolute zero. This property makes helium a valuable coolant in various scientific applications, such as superconducting magnets and cooling sensitive electronic equipments.
4.Density Variations: The densities of inert gases vary, with helium being the lightest and radon the heaviest. This variance in density makes inert gases useful in different contexts. Helium is commonly used to fill balloons due to its low density, causing the balloons to float. In contrast, radon, a radioactive noble gas, has applications in radiology and can be found in trace amounts in some geological formations.
5.Nonflammable and Non-Toxic: Inert gases are nonflammable and non-toxic, making them safe for various applications. This property is particularly crucial in confined spaces or environments where the presence of flammable or reactive substances could pose significant risks.
3.Cryogenics and Superconductivity: Helium, with its exceptionally low boiling point, is used as a cryogenic fluid in various applications. It is crucial for maintaining the low temperatures required for superconducting materials to exhibit their remarkable properties, such as zero electrical resistance. Superconducting magnets, used in MRI machines and particle accelerators, rely on helium cooling to achieve and maintain superconducting states.
4.Preservation of Sensitive Materials: Inert gases find applications in preserving materials that are sensitive to oxidation or degradation by exposure to air. Argon and nitrogen are commonly used to create controlled atmospheres for storing and preserving valuable artworks, historical documents, and delicate electronic components.
5.Deep Sea Diving: A mixture of helium and oxygen, known as heliox, is used in deep-sea diving to prevent nitrogen narcosis, a condition that occurs at great depths due to the increased pressure of nitrogen dissolved in the bloodstream. Helium's low solubility in blood reduces the risk of narcosis.
6.Space Exploration: Inert gases are found utility in space exploration. For instance, xenon is used as a propellant in ion thrusters, which provide efficient and precise propulsion for spacecraft. These thrusters use electric fields to accelerate ions (charged particles), resulting in high exhaust velocities and fuel efficiency.
7.Medical Imaging: Xenon, a noble gas with low toxicity, is used in medical imaging techniques like xenon-enhanced computed tomography (CT) scans. When inhaled by a patient, xenon provides improved imaging of lung ventilation and blood flow in the brain.
Applications of Inert Gases:-
1.Welding and Cutting: Argon and helium are widely used in gas tungsten arc welding (GTAW) and gas metal arc welding (GMAW), commonly known as TIG (tungsten inert gas) and MIG (metal inert gas) welding, respectively. These gases shield the welding area from atmospheric gases like oxygen and nitrogen, preventing contamination and ensuring high-quality welds. Helium is also used in laser cutting processes due to its ability to conduct heat away from the cutting zone significantly.
2.Lighting and Illumination: Neon, famous for its distinctive glow, is used in neon signs and indicator lights. These signs are created by introducing a small amount of neon gas into a sealed glass tube and is applied an electric current, which causes the gas to emit colored light. Helium, on the other hand, is employed in helium-neon (HeNe) lasers, which are widely used in scientific research, barcode scanners, and alignment purposes due to their coherent light output.
3.Cryogenics and Superconductivity: Helium, with its exceptionally low boiling point, is used as a cryogenic fluid in various applications. It is crucial for maintaining the low temperatures required for superconducting materials to exhibit their remarkable properties, such as zero electrical resistance. Superconducting magnets, used in MRI machines and particle accelerators, rely on helium cooling to achieve and maintain superconducting states.
4.Preservation of Sensitive Materials: Inert gases find applications in preserving materials that are sensitive to oxidation or degradation by exposure to air. Argon and nitrogen are commonly used to create controlled atmospheres for storing and preserving valuable artworks, historical documents, and delicate electronic components.
5.Deep Sea Diving: A mixture of helium and oxygen, known as heliox, is used in deep-sea diving to prevent nitrogen narcosis, a condition that occurs at great depths due to the increased pressure of nitrogen dissolved in the bloodstream. Helium's low solubility in blood reduces the risk of narcosis.
6.Space Exploration: Inert gases are found utility in space exploration. For instance, xenon is used as a propellant in ion thrusters, which provide efficient and precise propulsion for spacecraft. These thrusters use electric fields to accelerate ions (charged particles), resulting in high exhaust velocities and fuel efficiency.
7.Medical Imaging: Xenon, a noble gas with low toxicity, is used in medical imaging techniques like xenon-enhanced computed tomography (CT) scans. When inhaled by a patient, xenon provides improved imaging of lung ventilation and blood flow in the brain.
Orbital electronic configuration of Krypton atom :-

Future Prospects and Conclusion:-
The properties and applications of inert gases have far-reaching implications across various fields. As technology and scientific knowledge continue to advance, the role of inert gases is likely to expand. One avenue of exploration involves using noble gases for novel applications in nanotechnology and materials science. Additionally, ongoing research into advanced propulsion systems for space travel could lead to increased utilization of inert gases like xenon.
In conclusion, 'what is inert gas', is a class act of chemistry with their remarkable chemical stability and diverse physical properties, play indispensable roles in an array of scientific, industrial, and medical applications. From their use in welding and lighting to their significance in cryogenics and space exploration, these gases have truly earned their place as invaluable elements in our modern world. As our understanding....





Comments
There are no comments for this story
Be the first to respond and start the conversation.