Understanding the Fundamental Building Block of Matter
What Is an Atom?
Atoms are the bedrock of the material world. These tiny, invisible entities constitute the fundamental building blocks of matter, and their understanding is pivotal to comprehending the structure and behavior of the universe at the most fundamental level. In this lecture, we will delve into the nature of atoms, exploring their history, structure, and significance in our everyday lives.
**Historical Perspective**
The concept of the atom has a long and rich history, dating back to ancient civilizations. Early Greek philosophers, such as Democritus and Leucippus, proposed that matter was composed of indivisible particles they called "atomos." However, it wasn't until the 19th century that the idea of the atom as a scientific concept began to take shape.
John Dalton, an English chemist, is often credited with formalizing the atomic theory in the early 1800s. He proposed that all matter is made up of tiny, indivisible particles known as atoms, each with its own unique properties. This notion revolutionized the way we understand the composition of matter.
**The Structure of an Atom**
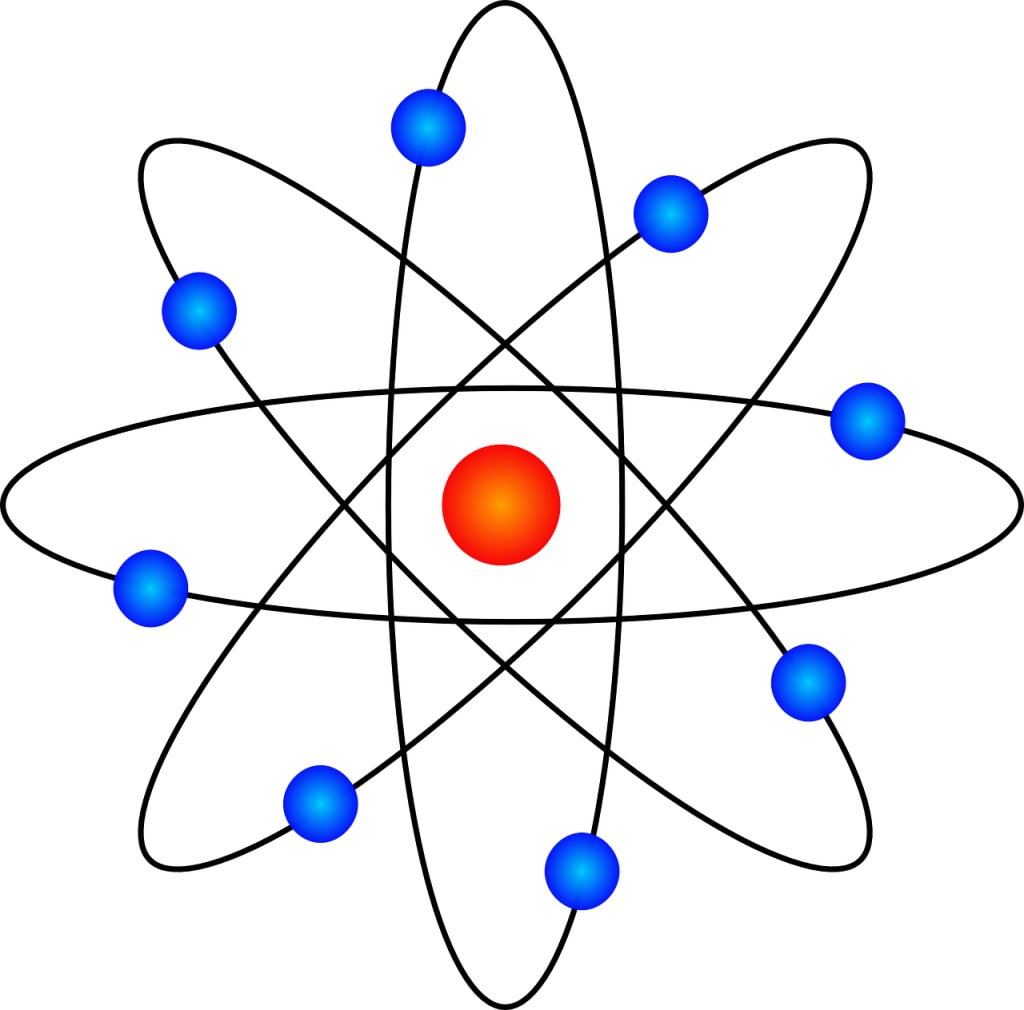
Atoms are incredibly small, with sizes on the order of angstroms (10^-10 meters). They consist of three main subatomic particles:
1. **Protons**: Positively charged particles located in the nucleus of the atom.
2. **Neutrons**: Electrically neutral particles found in the nucleus.
3. **Electrons**: Negatively charged particles that orbit the nucleus in specific energy levels or shells.
The nucleus is the central core of the atom, and it contains most of the atom's mass, primarily due to the protons and neutrons. Electrons orbit the nucleus in distinct energy levels, which are often visualized as concentric shells.
The number of protons in the nucleus determines an element's atomic number, which is unique for each element on the periodic table. For example, hydrogen has one proton, helium has two, and so on. The number of electrons in an atom is equal to the number of protons, resulting in a neutral charge.
**Atomic Bonding and Molecules**
Atoms rarely exist in isolation; they tend to combine to form molecules. The way atoms interact with one another depends on their electron arrangement. Atoms can share, donate, or accept electrons to achieve a stable electron configuration, which is the basis for chemical bonding.
There are two main types of chemical bonding:
1. **Covalent Bonding**: In covalent bonds, atoms share electrons to complete their outer electron shells. For example, in a water molecule (H2O), two hydrogen atoms share electrons with one oxygen atom, creating a stable molecule.
2. **Ionic Bonding**: In ionic bonds, one atom donates an electron (becoming positively charged) and another accepts it (becoming negatively charged). These oppositely charged ions are held together by electrostatic forces. An example is the sodium chloride (NaCl) molecule, where sodium donates an electron to chlorine.
**Isotopes and Atomic Mass**
Not all atoms of the same element are identical. Atoms can have different numbers of neutrons, resulting in isotopes. Isotopes of an element have the same number of protons but different numbers of neutrons. This variance in neutron count leads to differences in atomic mass.
For example, carbon-12 and carbon-14 are isotopes of carbon. Carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons. These isotopes have identical chemical properties but different atomic masses.
**The Significance of Atoms in Daily Life**
Atoms play a crucial role in our daily lives. Understanding atomic structure and behavior is the foundation of chemistry, and chemistry is all around us. Here are a few examples:
1. **Healthcare**: Medical imaging techniques like MRI rely on the behavior of atomic nuclei to create detailed images of the human body.
2. **Energy**: Understanding the atomic structure of materials is vital in the development of renewable energy sources and energy storage technologies.
3. **Materials Science**: Manipulating the arrangement of atoms in materials has led to innovations in electronics, polymers, and countless other fields.
4. **Environmental Science**: The study of atomic and molecular interactions informs environmental policies and helps combat pollution and climate change.
In conclusion, atoms are the essential constituents of matter, and their understanding has profound implications across various scientific disciplines and our everyday lives. As we continue to explore the frontiers of atomic science, we unlock new possibilities and deepen our understanding of the universe at its most fundamental level.






Comments (1)
Loved learning about atoms! Great work!