Molarity Definition: How to Calculate Concentration Like a Pro
Molarity Definition
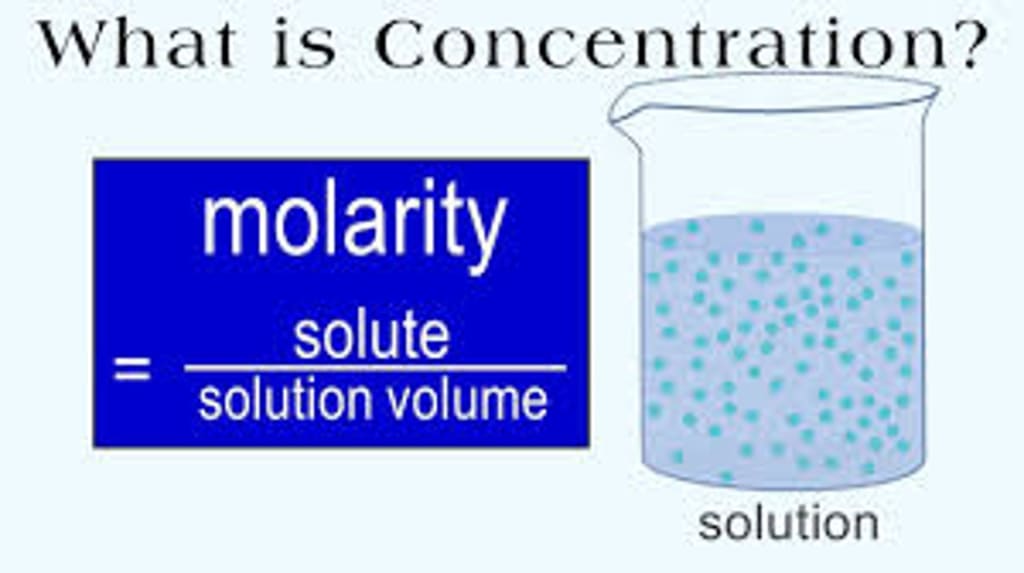
Are you struggling to understand the concept of molarity and how to calculate concentration? Don't worry, you're not alone. Molarity is a fundamental concept in chemistry, and it's essential to understand if you want to excel in the subject. In this article, we'll discuss molarity definition and explore how to calculate concentration like a pro. We'll also discuss the relationship between molarity and normality, and provide the formulas for both. So, let's dive in!
Table of Contents
What is Molarity?
Molarity Formula
How to Calculate Molarity
Units of Molarity
Molarity vs. Normality
Relationship Between Molarity and Normality
Normality Formula
How to Calculate Normality
Units of Normality
Molarity and Normality Examples
Factors That Affect Molarity
Limitations of Molarity
Applications of Molarity
Tips for Calculating Molarity
Conclusion
FAQs
What is Molarity?
Molarity is a measure of the concentration of a solution. It's defined as the number of moles of solute per liter of solution. In simpler terms, it tells us how much of a solute is dissolved in a given volume of a solution. The unit of molarity is mol/L, which means moles per liter.
Molarity Formula
The formula for molarity is simple and straightforward. It's given by:
Molarity (M) = Number of moles of solute (n) / Volume of solution (V)
where n is the number of moles of solute, and V is the volume of the solution in liters.
How to Calculate Molarity
To calculate the molarity of a solution, you need to know the number of moles of solute and the volume of the solution in liters. Let's consider an example to illustrate this.
Suppose you dissolve 0.5 moles of NaCl in 1 liter of water. What is the molarity of the resulting solution?
Using the molarity formula, we have:
Molarity (M) = Number of moles of solute (n) / Volume of solution (V)
= 0.5 mol / 1 L
= 0.5 M
Therefore, the molarity of the solution is 0.5 M.
Units of Molarity
The unit of molarity is mol/L, which means moles per liter. This unit is commonly used in chemistry to express the concentration of a solution. However, there are other units of concentration as well, such as percent by mass, percent by volume, and parts per million.
Molarity vs. Normality
Molarity and normality are both measures of concentration, but they differ in their definitions and applications. Molarity is defined as the number of moles of solute per liter of solution, whereas normality is defined as the number of equivalents of solute per liter of solution.
Relationship Between Molarity and Normality
The relationship between molarity and normality depends on the chemical reaction that is taking place. In general, the normality of a solution is equal to the molarity multiplied by the number of equivalents per mole of the solute. The formula for normality is given by:
Normality (N) = Molarity (M) x Number of equivalents per mole of solute
How to Calculate Normality
To calculate the normality of a solution, you need to know the molarity of the solute and the number of equivalents per mole of the solute. Let's consider an example to illustrate this.
Suppose you have a solution of HCl with a molarity of 0.1 M. What is the normality of the solution?
The number of equivalents per mole of HCl is 1, as it's a monoprotic acid. Therefore, using the normality formula, we have:
Normality (N) = Molarity (M) x Number of equivalents per mole of solute
= 0.1 M x 1
= 0.1 N
Therefore, the normality of the solution is 0.1 N.
Units of Normality
The unit of normality is N, which means equivalents per liter. This unit is commonly used in acid-base titrations to express the concentration of a solution.
Molarity and Normality Examples
Let's consider some examples to understand the relationship between molarity and normality.
Example 1:
Suppose you have a solution of H2SO4 with a molarity of 0.2 M. What is the normality of the solution?
H2SO4 is a diprotic acid, which means it can donate two protons per molecule. Therefore, the number of equivalents per mole of H2SO4 is 2.
Using the normality formula, we have:
Normality (N) = Molarity (M) x Number of equivalents per mole of solute
= 0.2 M x 2
= 0.4 N
Therefore, the normality of the solution is 0.4 N.
Example 2:
Suppose you have a solution of NaOH with a normality of 0.5 N. What is the molarity of the solution?
NaOH is a monoprotic base, which means it can accept one proton per molecule. Therefore, the number of equivalents per mole of NaOH is 1.
Using the molarity formula, we have:
Molarity (M) = Number of moles of solute (n) / Volume of solution (V)
= Normality (N) x Number of equivalents per mole of solute / Volume of solution (V)
= 0.5 N x 1 / V
= 0.5 V M
Therefore, the molarity of the solution is 0.5 V M.
Factors That Affect Molarity
The molarity of a solution can be affected by several factors, such as temperature, pressure, and the presence of other solutes. For example, if the temperature of a solution increases, the volume of the solution also increases, which can affect the molarity.
Limitations of Molarity
Although molarity is a useful measure of concentration, it has some limitations. For example, it assumes that the solute and solvent are completely mixed, which may not be the case in real-world scenarios. Also, it doesn't take into account the size or nature of the solute particles, which can affect the concentration.
Applications of Molarity
Molarity is a fundamental concept in chemistry and has many practical applications. For example, it's used in chemical reactions to determine the amount of reactants needed, and in the preparation of solutions with specific concentrations.
Tips for Calculating Molarity
Calculating molarity can be tricky, but with some practice, you can master it. Here are some tips to help you calculate molarity like a pro:
Make sure you have accurate measurements of the volume and mass of the solute and solvent.
Convert the mass of the solute to moles using the molar mass.
Calculate the volume of the solution in liters.
Use the molarity formula to calculate the molarity of the solution.
Double-check your calculations and units to avoid errors.
Conclusion
In conclusion, molarity is a measure of the concentration of a solution, and it's defined as the number of moles of solute per liter of solution. It's a fundamental concept in chemistry and has many practical applications. Normality is another measure of concentration that's defined as the number of equivalents of solute per liter of solution. The relationship between molarity and normality depends on the type of reaction that's taking place. Calculating molarity and normality requires accurate measurements and careful calculations. By following the tips provided in this article, you can calculate molarity like a pro.
FAQs
Q: What is the definition of normality?
Normality is a measure of the concentration of a solution, and it's defined as the number of equivalents of solute per liter of solution.
Q: What is the formula for normality?
The formula for normality is: Normality (N) = Molarity (M) x Number of equivalents per mole of solute.
Q: What is the unit of normality?
The unit of normality is N, which means equivalents per liter.
Q: What is the relationship between molarity and normality?
The relationship between molarity and normality depends on the chemical reaction that's taking place. In general, the normality of a solution is equal to the molarity multiplied by the number of equivalents per mole of the solute.
Q: What is the formula for molarity?
The formula for molarity is: Molarity (M) = Number of moles of solute (n) / Volume of solution (V)
About the Creator
Gaurav Mehra
I am a Chemistry Teacher at Infinity Learn and Teach students on YouTube for free. And here to help students through my blogs.






Comments
There are no comments for this story
Be the first to respond and start the conversation.