An Interesting Instance of Pertuzumab-Prompted Harmful Epidermal Necrolysis
"Pertuzumab Unveiled: A Shocking Encounter with Harmful Epidermal Necrolysis"
Unique
Pertuzumab is a designated treatment drug that is utilized in the administration of human epidermal development factor receptor 2 (HER2)- positive bosom disease and works by hindering the capacity of malignant growth cells to get development and multiplication signals. Poisonous epidermal necrolysis (TEN) is a serious cutaneous sign portrayed by far and wide erythema, putrefaction, and bullous separation of the skin including over 10% of the body surface region (BSA) and might be encouraged by an immunologic reaction to the organization of specific drugs. Nonetheless, TEN improvement as an outcome of HER2 inhibitor treatment has not been portrayed in the current writing. A 44-year-old female with a background marked by metastatic bosom malignant growth to the liver gave a diffuse rankling rash following a first-time organization of pertuzumab three days earlier. Her rash started as excruciating and pruritic rankles 12 hours after the last mixture of pertuzumab and advanced to include her arms, chest, crotch, and thighs with a positive Nikolsky sign. She was overseen steadily with high-portion steroids and allergy meds, and in spite of the fact that her clinic course was confounded by hypotension needing pressor help, she bit by bit made a full recuperation and was delivered to a restoration office.
Presentation
Pertuzumab (Perjeta) is a designated treatment that hinders human epidermal development factor receptor-2 (HER2), a protein found in HER2-positive bosom tumors. It works by obstructing the disease cells' capacity to get development signals, making it a significant therapy choice for HER2-positive bosom malignant growth [1].
Poisonous epidermal necrolysis (TEN) is an extreme skin response brought about by specific medications, including anti-infection agents, non-steroidal calming drugs (NSAIDs) [2], antiretroviral medications, and others. It is portrayed by erythema, corruption, and bullous separation of the skin and mucosal layers. The specific reason for TEN isn't completely perceived, yet it is accepted to include immunologic responses, receptive metabolites from drugs, and hereditary variables [3,4]. As far as we could possibly know, no case reports exist in the ongoing writing specifying TEN after organization of the HER2 inhibitor class of treatments.
We present a 44-year-old female with a background marked by HER2-positive left bosom malignant growth and liver metastases who created TEN after her most memorable portion of pertuzumab.
Case Show
A 44-year-old female gave to the crisis division a diffuse rankling rash following a first-time organization of pertuzumab three days earlier. Her clinical history was huge for triple-negative right bosom disease with biopsy-demonstrated liver metastases and estrogen receptor (emergency room) positive/progesterone receptor (PR) negative/HER-2 positive left bosom malignant growth.
Her home meds included trazodone, oxycodone, and ondansetron. Her underlying chemotherapy routine, which started two months preceding show, comprised of paclitaxel and trastuzumab. She got a sum of three portions of paclitaxel and one portion of trastuzumab during that time with no dermatologic incidental effects. Three days preceding show, the patient got a fourth portion of paclitaxel, a second portion of trastuzumab, and pertuzumab interestingly. The patient denied any known medication sensitivities, utilization of natural enhancements, or any progressions to her home drug routine.
Her rash started 12 hours after her last chemotherapy imbuement and began as difficult, pruritic rankles overlying her upper back and neck. Over the course of the following 24 hours, the rash spread to the arms, chest, midsection, crotch, and thighs. The rankles dynamically filled in size, and some of them burst suddenly, delivering serosanguineous liquid.
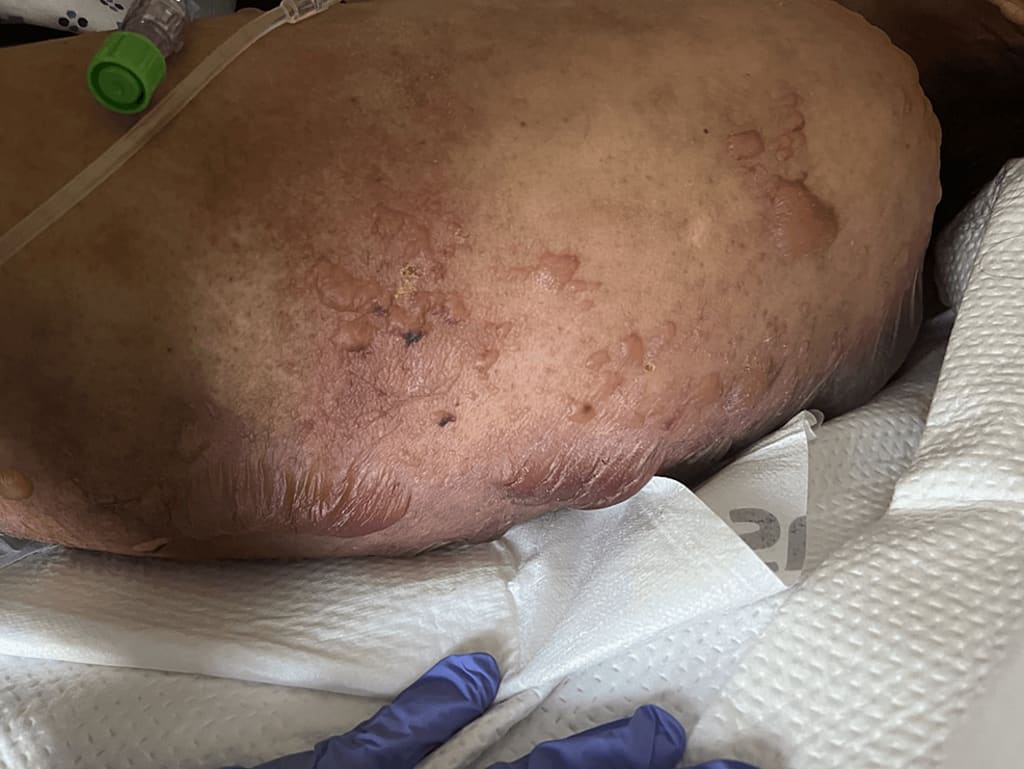
The patient introduced to the trama center with typical fundamental signs with the exception of a pulse of 114/min. An actual test uncovered an advanced female in noticeable misery. A skin test exhibited various vesicles and huge bullae encompassed by erythema (Figure 1).
Figure 1: Skin discoveries over upper back showing areas of bullae arrangement, rankling and erythema normal for harmful epidermal necrolysis
Skin-discoveries over-upper-back-showing-areas-of-bullae-development,- rankling and-erythema-normal for harmful epidermal-necrolysis
Figure 1: Skin discoveries over upper back showing areas of bullae development, rankling and erythema normal for harmful epidermal necrolysis
There were areas of bared skin. Nikolsky's sign was positive. The assessed body surface region (BSA) involved was 35%. Oral sores were not noticed.
A total blood count was huge for leukocytosis of 22,900/µL (reference range: 4,500-11,000/µL), thrombocytopenia of 100,000/µL (reference range: 150,000-400,000/µL), hemoglobin of 9.1 g/dL (reference range: 12-16 g/dL). Renal and liver capability tests were ordinary. CRP was 51.3 mg/L (reference range: <10.0 mg/L).
A skin biopsy uncovered epidermal separation with shallow epidermal rot and dyskeratotic keratinocytes predictable with TEN. She was at first moved to a basic consideration unit and began on IV steroids with dexamethasone (10 mg two times day to day) and diphenhydramine (25 mg like clockwork). Effective bacitracin was applied over the skin for disease prophylaxis, and twisted care with clean saline and Xeroform cloth was applied. IV hydration to reconstitute volume misfortune was continuous.
Her course in the basic consideration unit was additionally confounded by hypotension, with the differential finding including septic versus hypovolemic shock, for which she was begun on IV vasopressors, phenylephrine, and norepinephrine to keep a mean blood vessel pressure more noteworthy than 65 mmHg. She was hence moved to a devoted consume unit for additional administration. She kept on improving regarding TEN and was delivered to an actual restoration office.
Conversation
Stevens-Johnson condition (SJS) and TEN are extreme excessive touchiness responses with mucocutaneous contribution and a typical death pace of 1-5% in SJS and 25-35% in TEN [5]. Patients in the two circumstances present with skin erythema, delicacy, level abnormal objective sores, rankles, hemorrhagic disintegrations, and epidermal separation. Pressure applied to the skin causes epidermal separation (Nikolsky sign), however it isn't well defined for just SJS/TEN. SJS and TEN vary in the level of skin contribution, with SJS including under 10% of the complete body surface, though TEN includes over 30% of the all out body surface region. Contribution somewhere in the range of 10% and 30% is viewed as SJS-TEN cross-over.
These extreme touchiness responses are uncommon, with the mean assessed occurrences of SJS, SJS/TEN cross-over, and TEN of 9.2, 1.6, and 1.9 per million grown-ups each year in the US, separately [6]. A few known medications to incite SJS and TEN incorporate allopurinol, trimethoprim-sulfamethoxazole, other sulfonamide anti-infection agents, aminopenicillins, cephalosporins, quinolones, carbamazepine (CBZ), phenytoin, phenobarbital, and NSAIDs of the oxicam type [5]. Certain diseases, for example, Mycoplasma pneumoniae and the herpes simplex infection, can likewise initiate this response. A writing survey somewhere in the range of 1968 and 2012 of the Food and Medication Organization Unfavorable Occasion Detailing Framework (FAERS) revealed critical signs of SJS and TEN to a few anticancer medications, including bendamustine, busulfan, chlorambucil, fludarabine, lomustine, and procarbazine [7,8]. How various drugs and contaminations can prompt SJS and TEN is muddled, yet cytotoxic T lymphocytes (CD8+), FasL, and granulysin are accepted to be answerable for scattered keratinocyte apoptosis in SJS/TEN [5].
Our patient had TEN including roughly 35% of her body surface region following her most memorable openness to pertuzumab, a monoclonal immunizer going about as an inhibitor of HER2 dimerization, for her therapy of metastatic bosom malignant growth. Pertuzumab is utilized in blend with trastuzumab and docetaxel for the therapy of HER2+ metastatic bosom disease. Pertuzumab is by and large very much endured, for certain incidental effects like looseness of the bowels, sickness, retching, asthenia, and a rash. Skin rashes, as revealed in most clinical preliminaries, are poor quality (grade 1 or 2) with a papulopustular (acneiform) aggregate [1]. The concentrate likewise revealed that growth types appear to impact the gamble of rash frequency in patients who are on pertuzumab, with bosom, ovarian, fallopian tube, and peritoneal disease having a higher rate of rashes than prostate disease, and the general occurrence of rash with pertuzumab in bosom malignant growth was 28.5% (95% CI 25.5-31.7%).
A significant thought to make in this specific case is the unmistakable transient relationship between the organization of pertuzumab and the improvement of our patient's side effects, as most instances of TEN prompted by a culpable medicine have been contemplated to have a middle idleness time between the start of purpose and the beginning of TEN of under about a month [9].
Hereditary inclination has been displayed to interface SJS/TEN with specific human leukocyte antigens (HLA) and drugs. CBZ-prompted SJS/TEN is connected with HLA-B*15:02 in Asian populaces, remembering those for China, Thailand, Malaysia, and India, however there is no relationship with HLA-B*15:02 in Japanese, Korean, or European populaces [10]. Then again, CBZ-prompted SJS/TEN in Japanese, Korean, and European populaces is connected to HLA-A*31:01. HLA affiliation is subsequently not a sole figure the improvement of SJS/TEN yet appears to contrast among changed identities too. Different medications like allopurinol are connected to HLA-B*58:01 in Taiwanese, Japanese, Korean, Thai, and European people, while abacavir is related with HLA-B*57:01. Screening rules have been executed to forestall SJS/TEN advancement in certain drugs [11]. Considering that our patient is of Indian legacy, a further investigate the connection between HLA affiliation and the improvement of SJS/TEN in various disease medications might be gainful for future exploration.
The executives of TEN/SJS fundamentally comprises of prompt withdrawal of thought drugs and steady consideration. Keeping up with electrolyte balance and appropriate injury care are significant asof the executives. Early utilization of corticosteroids inside 24-48 hours of side effect beginning has been displayed to have a decent result, as has the utilization of other immunomodulators like IVIG, cyclosporine, tacrolimus, and cyclophosphamide [12]. The endurance rate is demonstrated to be fundamentally higher in patients who were moved to the consume unit in something like seven days after sickness beginning [5].
Ends
This case exhibits the expected connection between harmful epidermal necrolysis and the chemotherapy drug pertuzumab. Albeit interesting, poisonous epidermal necrolysis can be perilous and requires brief finding and the board. Early withdrawal of thought drugs, steady consideration, and the utilization of corticosteroids and other immunomodulators can assist with dealing with this condition. Further examination on the connection between human leukocyte antigen affiliation and the improvement of poisonous epidermal necrolysis with various disease medications might be valuable later on, especially for patients of various identities.
References
1.Drucker AM, Wu S, Darn CT, Lacouture ME: Chance of rash with the counter HER2 dimerization neutralizer pertuzumab: a meta-investigation. Bosom Disease Res Treat. 2012, 135:347-54. 10.1007/s10549-012-2157-7
2.Kameshwari JS, Devde R: A case report on poisonous epidermal necrolysis with etoricoxib. Indian J Pharmacol. 2015, 47:221-3. 10.4103/0253-7613.153436
3.Bazine A, Fetohi M, Namad T, El Benaye J, Ennouhi Mama, Mahfoud T, Ichou M: Harmful epidermal necrolysis in a female with metastatic bosom disease treated with vinorelbine. Ann Consumes Fire Debacles. 2017, 30:261-3.
4.Halevy S, Ghislain PD, Mockenhaupt M, et al.: Allopurinol is the most widely recognized reason for Stevens-Johnson disorder and poisonous epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008, 58:25-32. 10.1016/j.jaad.2007.08.036
5.Harr T, French LE: Harmful epidermal necrolysis and Stevens-Johnson disorder. Orphanet J Interesting Dis. 2010, 5:39. 10.1186/1750-1172-5-39
6.Hsu DY, Brieva J, Silverberg NB, Silverberg JI: Bleakness and mortality of Stevens-Johnson disorder and poisonous epidermal necrolysis in US grown-ups. J Contribute Dermatol. 2016, 136:1387-97. 10.1016/j.jid.2016.03.023
7.Rosen AC, Balagula Y, Raisch DW, et al.: Perilous dermatologic antagonistic occasions in oncology. Anticancer Medications. 2014, 25:225-34. 10.1097/CAD.0000000000000032
8.Hasegawa A, Abe R: Late advances in overseeing and figuring out Stevens-Johnson condition and poisonous epidermal necrolysis. F1000Res. 2020, 9:10.12688/f1000research.24748.1
9.Mockenhaupt M: The ongoing comprehension of Stevens-Johnson disorder and harmful epidermal necrolysis. Master Fire up Clin Immunol. 2011, 7:803-13; test 814-5. 10.1586/eci.11.66
10.Zagouri F, Sergentanis TN, Chrysikos D, et al.: Pertuzumab in bosom malignant growth: a precise survey. Clin Bosom Malignant growth. 2013, 13:315-24. 10.1016/j.clbc.2013.05.002
11.FitzGerald JD, Dalbeth N, Mikuls T, et al.: 2020 American School of Rheumatology Rule for the administration of gout. Joint pain Care Res (Hoboken). 2020, 72:744-60. 10.1002/acr.24180
12.Kumar R, Das A, Das S: The executives of Stevens-Johnson condition harmful epidermal necrolysis: looking past rules!. Indian J Dermatol. 2018, 63:117-24. 10.4103/ijd.IJD_583_17





Comments
There are no comments for this story
Be the first to respond and start the conversation.