Introduction
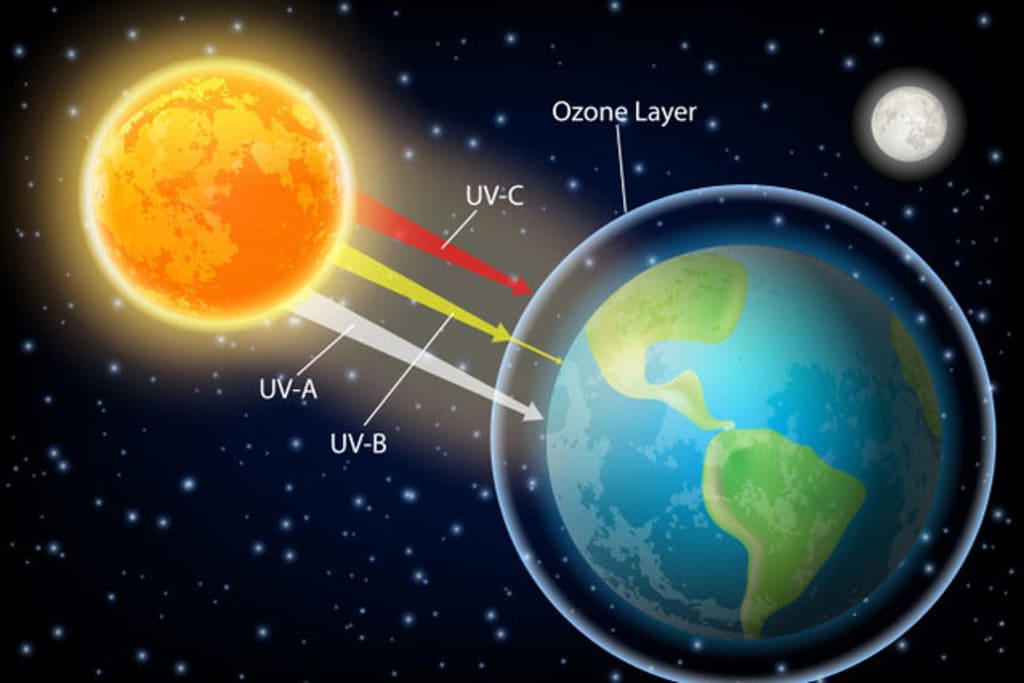
The ozone layer is a thin layer in Earth's atmosphere that contains a high concentration of ozone (O3) molecules. It is located in the stratosphere, at an altitude of about 10 to 50 kilometers (6 to 30 miles) above the Earth's surface.
Ozone is a highly reactive and unstable molecule that is formed when ultraviolet (UV) radiation from the sun breaks apart molecular oxygen (O2) in the atmosphere. The resulting oxygen atoms then react with other oxygen molecules to form ozone.
The ozone layer plays a critical role in protecting life on Earth from the harmful effects of UV radiation. UV radiation can cause skin cancer, cataracts, and other health problems in humans, as well as damage crops and other living organisms. The ozone layer absorbs much of the incoming UV radiation, preventing it from reaching the Earth's surface.
In recent decades, scientists have discovered that human activities, such as the release of chlorofluorocarbons (CFCs) and other ozone-depleting substances, have damaged the ozone layer. This has led to a thinning of the ozone layer, especially over the polar regions, known as the "ozone hole." The international community has taken action to phase out the production and use of ozone-depleting substances, and the ozone layer is slowly recovering, although it is expected to take several decades to fully recover.
Ozone layer Depletion
Ozone layer depletion refers to the thinning of the ozone layer in the stratosphere due to the breakdown of ozone molecules by chemicals like chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and other ozone-depleting substances (ODS).
These chemicals are released into the atmosphere by human activities, such as industrial processes, refrigeration, and air conditioning. Once released, they can remain in the atmosphere for many years and can travel long distances before breaking down.
When these chemicals reach the stratosphere, they are broken down by UV radiation, releasing chlorine and bromine atoms. These atoms then react with ozone molecules, breaking them down into oxygen molecules and reducing the concentration of ozone in the atmosphere.
The thinning of the ozone layer has serious consequences for human health and the environment. UV radiation can cause skin cancer, cataracts, and other health problems in humans and animals. It can also damage crops and other living organisms, and affect the composition of the atmosphere and climate.
In response to these concerns, the international community has taken action to address ozone depletion by phasing out the production and use of ozone-depleting substances under the Montreal Protocol, a global treaty signed in 1987. Thanks to these efforts, the ozone layer is slowly recovering, and it is expected to return to pre-1980 levels by the middle of the 21st century.
How can we protect the Ozone Layer
There are several ways that we can protect the ozone layer:
1. Reduce the use of ozone-depleting substances: The most important step in protecting the ozone layer is to reduce the production and use of ozone-depleting substances such as chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and halons. This can be achieved through the use of alternative chemicals and technologies that are more environmentally friendly.
2. Proper disposal of ozone-depleting substances: Proper disposal of refrigerators, air conditioners, and other products containing ozone-depleting substances can prevent them from releasing these harmful chemicals into the atmosphere.
3. Support international efforts to protect the ozone layer: Governments can support international efforts to protect the ozone layer by enforcing regulations and laws that restrict the production and use of ozone-depleting substances, and by contributing to international funds that support projects aimed at protecting the ozone layer.
4. Use energy-efficient appliances: Energy-efficient appliances such as refrigerators, air conditioners, and light bulbs can reduce the need for these products and reduce the demand for ozone-depleting substances.
5. Spread awareness about ozone depletion: Educating the public about the importance of protecting the ozone layer and the harmful effects of ozone depletion can help raise awareness and encourage individuals and communities to take action.
By taking these steps, we can help protect the ozone layer and ensure a safer and healthier planet for ourselves and future generations.
About the Creator
Love The Green
Welcome to my page,I hope to share my experiences, insights, and knowledge with fellow nature enthusiasts.Together, we can celebrate the wonders of the natural world, and work to protect and preserve it for future generations to enjoy🌿🌲






Comments
There are no comments for this story
Be the first to respond and start the conversation.