The US and UK argue over the decision to approve the Pfizer vaccine
Dr Anthony Fauci expressed doubts about Britain's decision to approve the Pfizer vaccine, later withdrawing his comments.

Dr. Anthony Fauci expressed doubts about Britain's decision to approve the Pfizer vaccine, later withdrawing his comments.
On 3/12, Gavin Williamson, UK Minister of Education expressed his delight at the country's decision to approve the Pfizer vaccine. "Obviously our health regulators work best. Better than France, much better than Belgium and America," he affirmed.
He praised the officials and the country as a whole: "This does not surprise me. Our country is far superior to other countries."
However, Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, expressed doubts about the British decision. According to Fauci, UK authorities approved vaccines faster simply because they did not review data as carefully as the Food and Drug Administration (FDA).
"We have the gold standard in terms of access to vaccines. You don't have that thoroughness so they are a few days ahead," he said.
Later, when appearing on British television, Fauci seemed to want to withdraw his statements. In an interview with the BBC, he said: "I am very confident in what the UK does, both in terms of science and the stance of the regulator". He also did not judge the country because he approved the Pfizer vaccine so quickly.
"We (America) do things a little differently, that's all. It's not better, it's not worse, it's just different," he added.
Dr. Fauci said the politicization of the pandemic in the United States has made regulators more cautious in order to preserve public trust. He admits the stress in this country makes many people shy when it comes to vaccines. "Vaccine nationalism has no place in Covid-19 and other public health issues," said Jeremy Farrar, director of the Wellcome Trust and UK scientific advisor. from this terrible pandemic ".
Dr. Fauci is one of the most influential figures in the epidemic in America. President-elect Joe Biden also announced that Mr. Fauci would play a medical center role in the new administration.
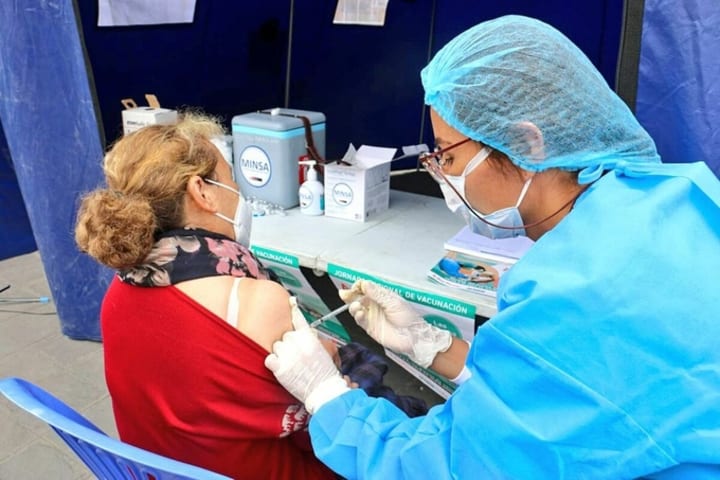
Trial complete by Pfizer vaccine, 95% effective
Pharmaceutical company Pfizer has completed the test of the BNT162b2 vaccine, the product is 95% effective, without leaving significant side effects.
In a statement dated November 18, the company representative said this was the first complete result of a late-stage test. The data showed that the vaccine, which prevents mild and severe forms of Covid-19, is 94% effective in the elderly, who are susceptible to getting worse with nCoV infection.
Pfizer plans to submit an application for urgent approval to the US Food and Drug Administration (FDA) within the next few days, expecting to soon launch an effective vaccine that will help end the pandemic.
The results of the test were released less than a year after the scientists began working on the vaccine, breaking all development speed records. This process has been going on for many years, even decades.
Pfizer's phase three trial was conducted with nearly 44,000 volunteers, divided into two groups. Half of the vaccine was given, and the other half used a placebo. Experts then wait to see how many people are infected with nCoV. To date, the company said that out of 170 volunteers infected with Covid-19, only eight were vaccinated, and the remaining 162 were taking a placebo. Out of 10 cases with severe symptoms, only one vaccinated.
Pfizer and German partner BioNTech say the vaccine's effectiveness is consistent across age, race and ethnicity. The most common and serious side effect is fatigue. 3.7% of the volunteers said they were tired after the second shot of the vaccine. 2% reported symptoms of a headache. Older adults experience fewer side effects.
Akiko Iwasaki, an immunologist at Yale University, said: "This is quite amazing. We understand that the flu vaccine is very difficult to be effective in this age group. So the 94% is really impressive."
Health officials said the first doses of the vaccine are likely to be for people who are susceptible to disease or for frontline physicians at high risk of exposure to the virus. Both Pfizer and Moderna acknowledge the pandemic tension has accelerated vaccine development and testing, as volunteers are increasingly infected with nCoV.
After Pfizer, Russia also announced that the Sputnik vaccine was 92% effective, but did not provide detailed data. Pharmaceutical company Moderna a few days ago also announced its vaccine is 94.5% effective.
About the Creator
Phát Nguyễn Đức
Just a young teenager want to express his skill in writing.





Comments
There are no comments for this story
Be the first to respond and start the conversation.